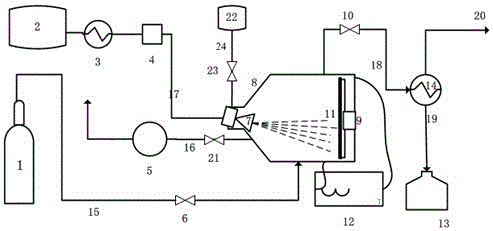
A method and device for catalytic hydrogen production suitable for hydrogen energy vehicles
A technology for automobiles and catalysts, which is applied in the field of catalytic hydrogen production by liquid organic hydrides. It can solve the problems of reduced dehydrogenation effects, expensive catalysts, and bulky reaction devices, so as to reduce reaction costs, simplify the hydrogen production process, and improve reaction efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]Take 12 g of Raney nickel catalyst, wash 3 times with absolute ethanol to replace the moisture therein, and press the catalyst into a circular sheet, and fix it on the rotating electromagnetic heating plate at the bottom of the reactor; turn on the vacuum pump to turn the reactor Vacuumize, turn off the vacuum pump, open the nitrogen bottle, feed nitrogen into the reactor, turn on the rotating electromagnetic heating plate under the protection of nitrogen, and slowly raise the temperature of the catalyst at a rate of 10°C / min to remove the bound moisture and Anhydrous ethanol, when the temperature rises to 160°C, the catalyst has been dried, at this time stop nitrogen, open the liquid organic hydride cooler to cool the cyclohexane to 6°C; set the dehydrogenation reaction temperature to 381°C, and react Electromagnetically heat the bottom of the reactor to the dehydrogenation reaction temperature, turn on the temperature control speed changer, keep the rotation speed of th...
Embodiment 2
[0029] Take 20 g of the catalyst Raney nickel, wash 3 times with absolute ethanol to replace the water therein, and press the catalyst into a circular sheet, and fix it on the rotating electromagnetic heating plate at the bottom of the reactor; turn on the vacuum pump to turn the reactor Vacuumize, turn off the vacuum pump, open the nitrogen bottle, feed nitrogen into the reactor, turn on the rotating electromagnetic heating plate under the protection of nitrogen, and slowly raise the temperature of the catalyst at a rate of 10°C / min to remove the bound moisture and Anhydrous ethanol, when the temperature rises to 160°C, the catalyst has been dried, at this time stop nitrogen, open the liquid organic hydride cooler to cool the cyclohexane to 6°C; set the dehydrogenation reaction temperature to 363°C, and react Electromagnetically heat the bottom of the reactor to the dehydrogenation reaction temperature, turn on the temperature control variable speed instrument, keep the rotati...
Embodiment 3
[0031] Take 20 g of the catalyst Raney nickel, wash 3 times with absolute ethanol to replace the water therein, and press the catalyst into a circular sheet, and fix it on the rotating electromagnetic heating plate at the bottom of the reactor; turn on the vacuum pump to turn the reactor Vacuumize, turn off the vacuum pump, open the nitrogen bottle, feed nitrogen into the reactor, turn on the rotating electromagnetic heating plate under the protection of nitrogen, and slowly raise the temperature of the catalyst at a rate of 10°C / min to remove the bound moisture and Anhydrous ethanol, when the temperature rises to 160°C, the catalyst has been dried, at this time, the nitrogen flow is stopped, and the liquid organic hydride cooler is opened to cool the cyclohexane to 6°C; the dehydrogenation reaction temperature is set to 371°C, and the reaction Electromagnetically heat the bottom of the reactor to the dehydrogenation reaction temperature, turn on the temperature control variabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com