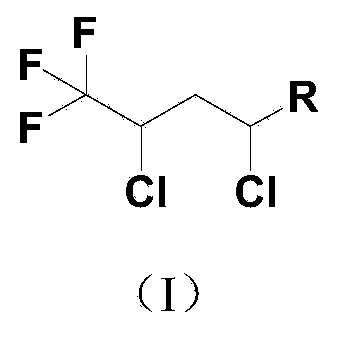
Preparation method of 2,4-dichloro-1,1,1-trifluorobutane derivative
A technology of trifluorobutane and its derivatives, which is applied in the field of preparation of 2,4-dichloro-1,1,1-trifluorobutane derivatives, can solve the problems of three wastes in the reaction, and achieve the improvement of catalytic efficiency, The effect of less three wastes and mature industrial production technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 1.4 grams of copper chloride and 3.2 grams of 2,2-bipyridine to a 500 mL titanium alloy belt stirred autoclave, dissolve them in 20 mL of acetonitrile, replace the air in the kettle with nitrogen, and inject 312 grams of R123 and 63 grams of benzene with nitrogen pressure. Ethylene, reaction temperature 60°C, reaction time 12 hours, after the reaction, the crude product was distilled at atmospheric pressure to remove R123 and acetonitrile, and then recycled, continued vacuum distillation to collect 1,1,1-trifluoro-2,4-dichloro- 4-butylbenzene, conversion rate 100%, selectivity 96.2%.
Embodiment 2~7
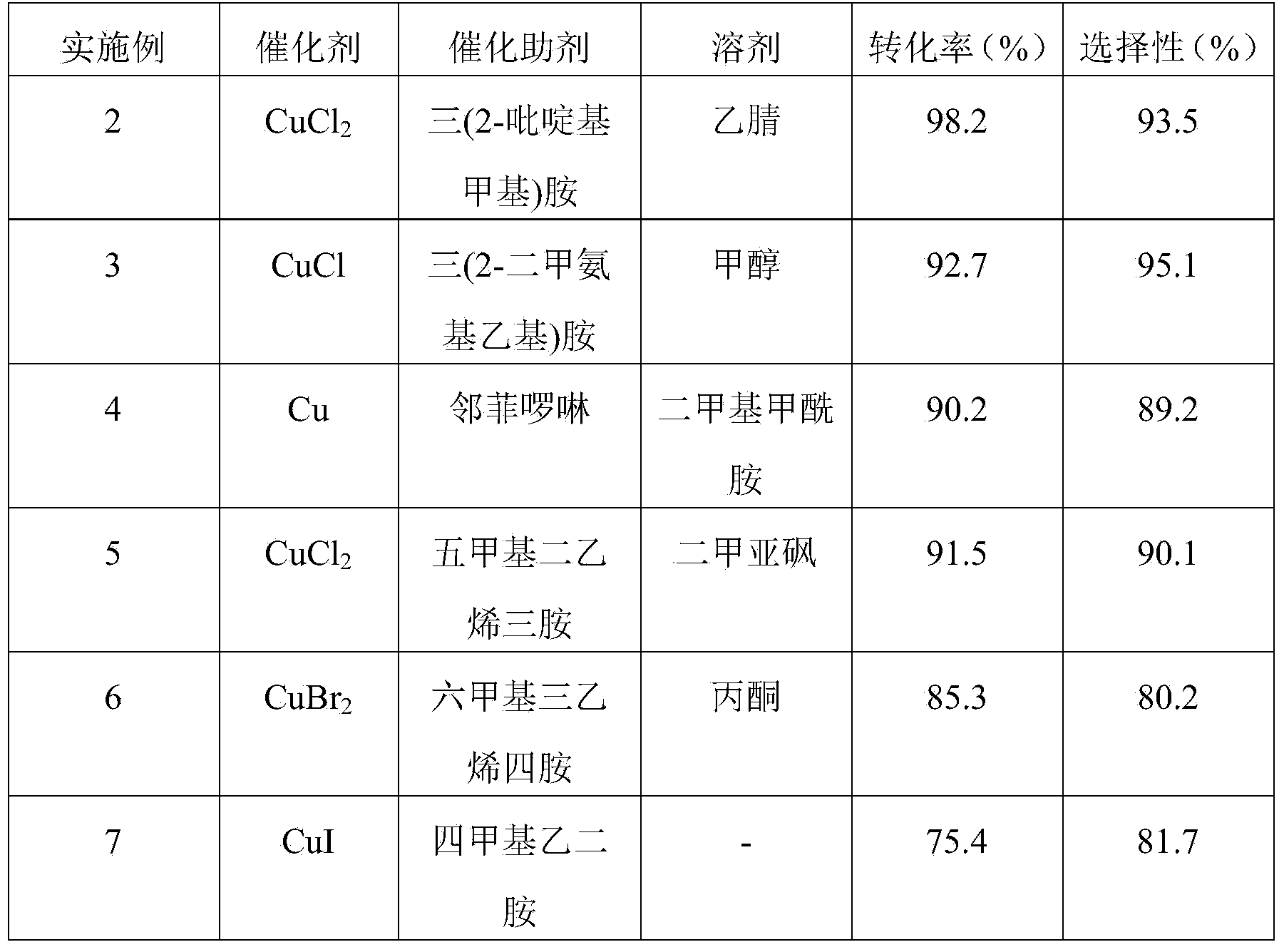
[0023] Examples 2-7 Prepare 1,1,1-trifluoro-2,4-dichloro-4-butylbenzene according to the same preparation method as in Example 1, except that the reaction catalyst in Example 1 is chlorine Copper chloride, and copper element, cupric chloride, cuprous chloride, cupric bromide or cuprous iodide respectively in embodiment 2~7. The reaction results of Examples 2-7 are shown in Table 1.
[0024] Table 1 Catalyst and Catalytic Auxiliary Effect
[0025]
Embodiment 8~13
[0027] Examples 8-13 Prepare 2,4-dichloro-1,1,1-trifluorobutane derivatives according to the same preparation method as in Example 1, except that styrene is used as raw material in Example 1, and The raw materials used in Examples 8-13 are respectively naphthalene ethylene, methyl styrene, n-hexene, isooctene, trifluoropropene and propenyl alcohol. The reaction results of Examples 8-13 are shown in Table 2.
[0028] Table 2 The reaction results of different kinds of alkenes
[0029] Example
[0030] 11
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com