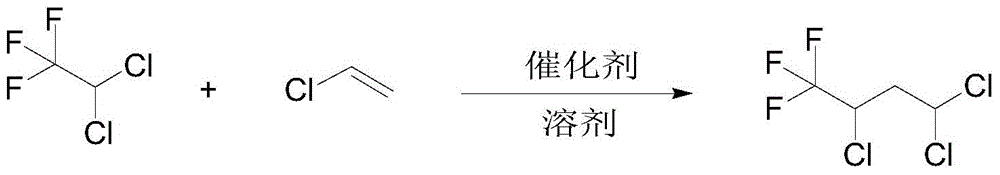
A kind of preparation method of 2,4,4-trichloro-1,1,1-trifluorobutane
A technology of trifluorobutane and trifluoroethane, which is applied in the field of preparation of hydrofluorochloroalkanes, can solve the problems of difficult availability and high price of trifluoropropene, and achieves the effects of high price and mature industrial production technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 1.4 grams of copper chloride and 3.2 grams of 2,2-bipyridine to a 500 mL titanium alloy belt stirred autoclave, dissolve them in 20 mL of dimethyl sulfoxide, replace the air in the kettle with nitrogen, and inject 312 grams of R123 with nitrogen gas and 63 grams of vinyl chloride, the reaction temperature is 120°C, the reaction pressure is 1.2MPa, and the reaction time is 12 hours. After the reaction, the crude product is removed by atmospheric distillation to remove R123, R123 is recycled, and the vacuum distillation is continued to collect 2,4,4-trichloro- 1,1,1-Trifluorobutane, the conversion rate is 91.3%, and the selectivity is 87.6%.
[0017] Product structure characterization:
[0018] 1 H-NMR (500MHz, CDCl 3 ): δ2.66(m,1H), δ2.82(m,1H), δ4.37(m,1H), δ5.92(q,1H)
[0019] 13 C-NMR (500MHz, CDCl 3 ): δ44.52(s,1C), δ54.31(q,1C), δ68.53(s,1C), δ124.48(q,1C)
[0020] 19 F-NMR (500MHz, CDCl 3 ): δ-74.66(s,3F)
Embodiment 2~6
[0022] Examples 2-6 Prepare 2,4,4-trichloro-1,1,1-trifluorobutane according to the same preparation method as in Example 1, except that the reaction 2,2-difluorobutane in Example 1 Chlorine 1,1,1-trifluoroethane: vinyl chloride ratio is 2:1, while the telomerization ratios in Examples 2-6 are 1:1, 3:1, 5:1, 7:1, 10: 1. The reaction results of Examples 2-6 are shown in Table 1.
[0023] Table 1 Effect of telomerization ratio
[0024] Example
Embodiment 7~18
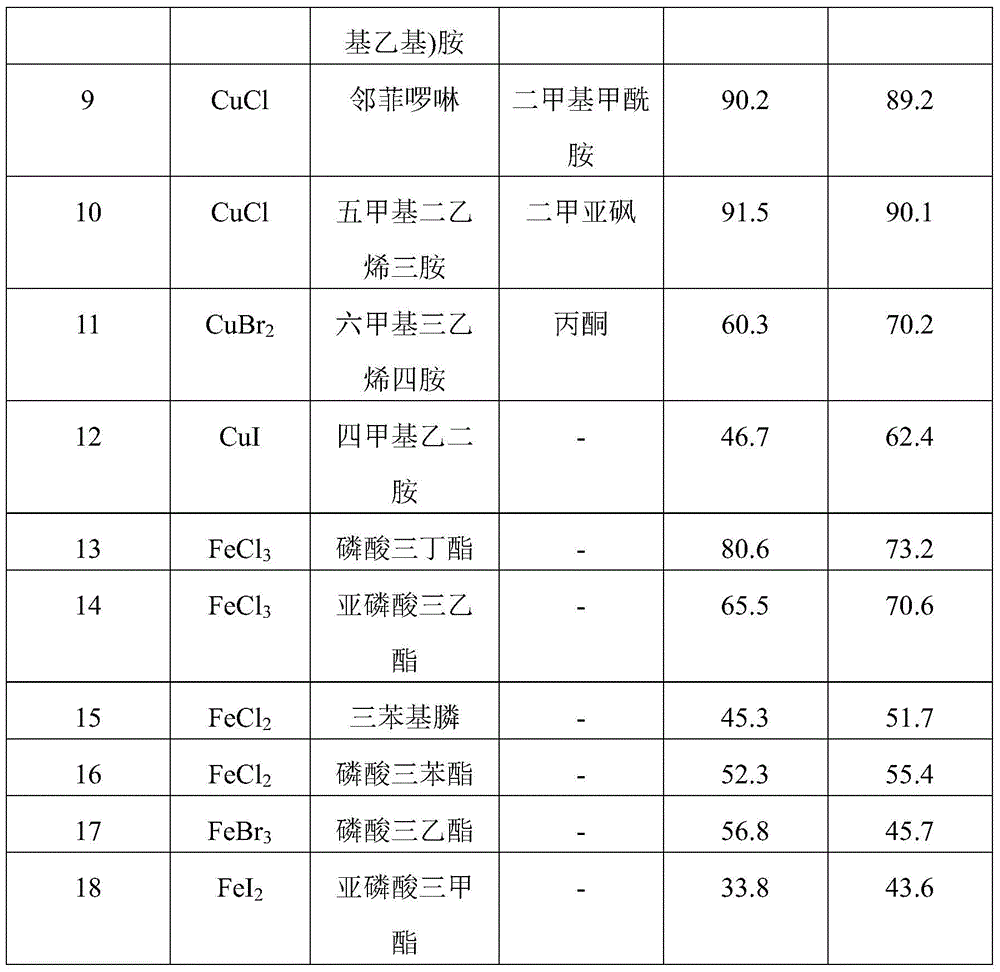
[0026] Examples 7-18 Prepare 2,4,4-trichloro-1,1,1-trifluorobutane according to the same preparation method as in Example 1, except that the reaction catalyst in Example 1 is copper chloride , and respectively ferric chloride, ferrous chloride, cupric chloride or cuprous chloride in the embodiment 7~18, the reaction can be carried out without adding solvent. The reaction results of Examples 7-18 are shown in Table 2.
[0027] Table 2 Catalyst and Catalytic Auxiliary Effect
[0028]
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com