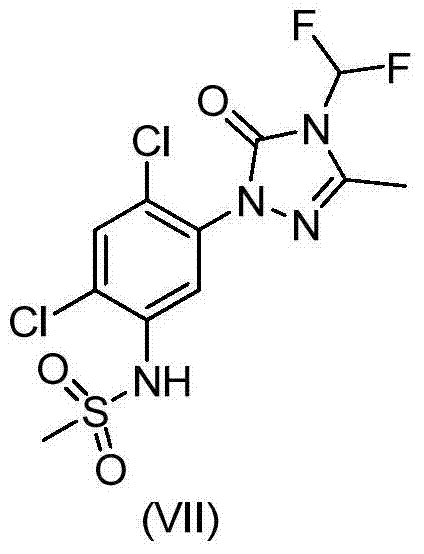
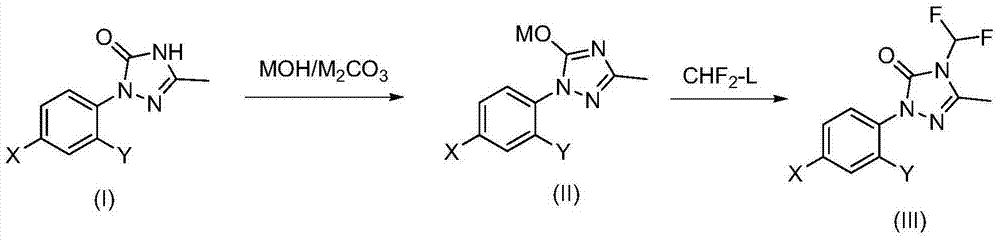
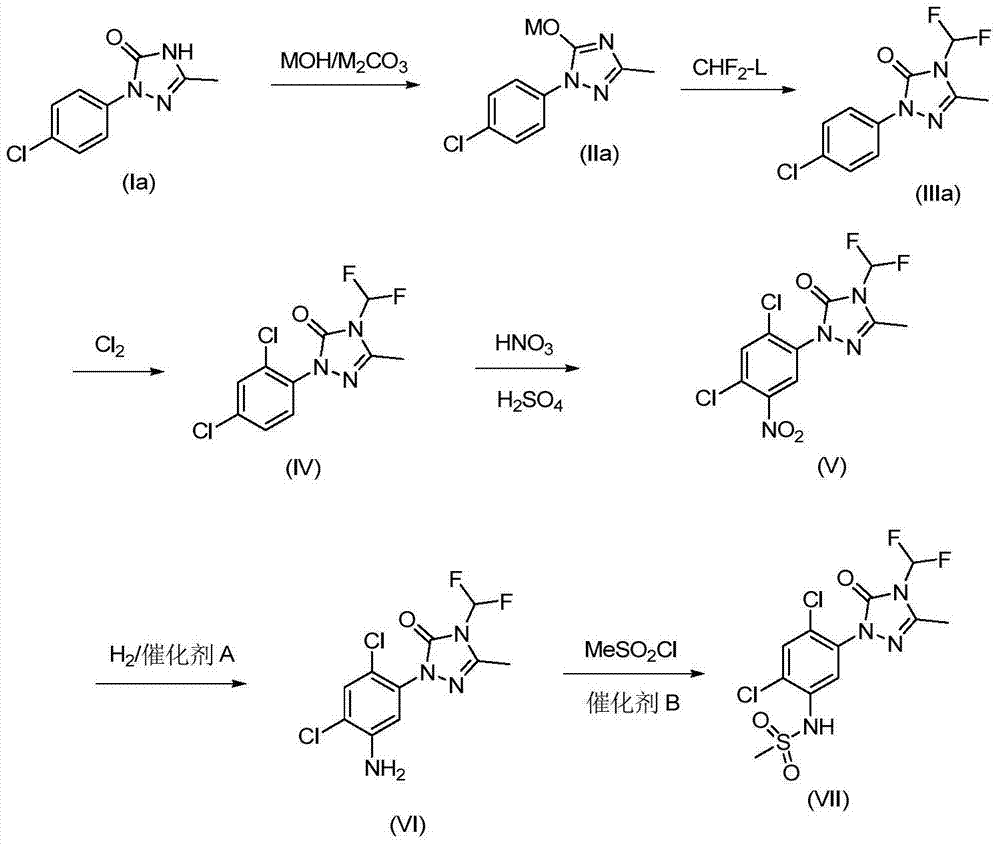
Method for synthesizing difluoro methyl triazoline-ketone and sulfentrazone
A technology for difluoromethyltriazolinone and difluoromethylation, which is applied in the field of synthesis of organic fluorine compounds, can solve the problems of affecting product quality and yield, generating formamide impurities, poor atom economy and the like, and achieves operation The effect of convenience, less side reactions and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Add 17.9g 1-phenyl-3-methyl-1,2,4-triazolin-5-one, 115mL toluene, 6.9g potassium carbonate, 6.2g potassium hydroxide and 22mL to a 500mL reaction flask water, heated and refluxed for dehydration. Add 130mL DMSO, heat up and distill toluene to an internal temperature of 145-150°C. Pass difluorochloromethane until the reaction raw material content is lower than 0.5%. Stop the reaction, drop to room temperature and filter with suction, and wash the filter cake with DMSO. The filtrate was precipitated, slurred with water, filtered, rinsed with water, and dried to obtain 20.1 g of the product.
Embodiment 2
[0036] Example 2: Add 15g of 1-phenyl-3-methyl-1,2,4-triazolin-5-one, 45mL of toluene, 6.4g of potassium carbonate, 5.3g of potassium hydroxide and 12mL of water into a 500mL reaction flask , heating and reflux dehydration. Add 120mL of DMF, heat up and distill the toluene to an internal temperature of 145-150°C. Pass difluorochloromethane until the reaction raw material content is lower than 0.5%. Stop the reaction, drop to room temperature and filter with suction, and wash the filter cake with DMF. The filtrate was precipitated, slurred with water, filtered, rinsed with water, and dried to obtain 17.5 g of the product.
Embodiment 3
[0037] Example 3: Add 21.4g 1-(4-chlorophenyl)-3-methyl-1,2,4-triazolin-5-one, 115mL toluene, 10.6g potassium carbonate, 6.2g to a 500mL reaction flask Potassium hydroxide and 23mL of water were heated and refluxed for dehydration. Add 165mL DMSO, heat up and distill toluene to an internal temperature of 145-150°C. Pass difluorochloromethane until the reaction raw material content is lower than 0.5%. Stop the reaction, drop to room temperature and filter with suction, and wash the filter cake with DMSO. The filtrate was desolvated, slurred with water, filtered, rinsed with water, and dried to obtain 24 g of the product. 1 HNMR (500MHz, DMSO-d6) 7.84 (2H, dd, J = 9Hz), 7.56 (2H, dd, J = 9Hz), 7.52 (1H, t, J = 57Hz), 2.42 (3H, s); MS ( [M] + ): 259.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com