A kind of hyperbranched polysiloxane containing macrocyclic structure and its synthetic method
A polysiloxane and a synthesis method technology, applied in the field of hyperbranched polysiloxane synthesis, can solve the problems of complex cross-linking and synthesis process of synthesis technology, small pores between molecular branches, limited application scope, etc., and achieve product purification. The process is simple and easy, the synthesis efficiency is high, and the effect of cross-linking is not easy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of Hyperbranched Polysiloxane Containing Macrocyclic Structure with Phenyl and Methyl Side Groups
[0049] At 80°C, add 80 grams of 1,1,3,3-tetramethyl-5,7-diphenylcyclotetrasilane and 0.08 grams of water into a 500-milliliter two-necked flask containing 0.008 grams of palladium chloride and 300 milliliters of tetrahydrofuran , and stir for 10 minutes. Activated carbon was added to remove palladium chloride, filtered, the solvent was evaporated with a rotary evaporator, and finally vacuum-dried to obtain a white solid with a yield of 85% and a branching degree of 90%.
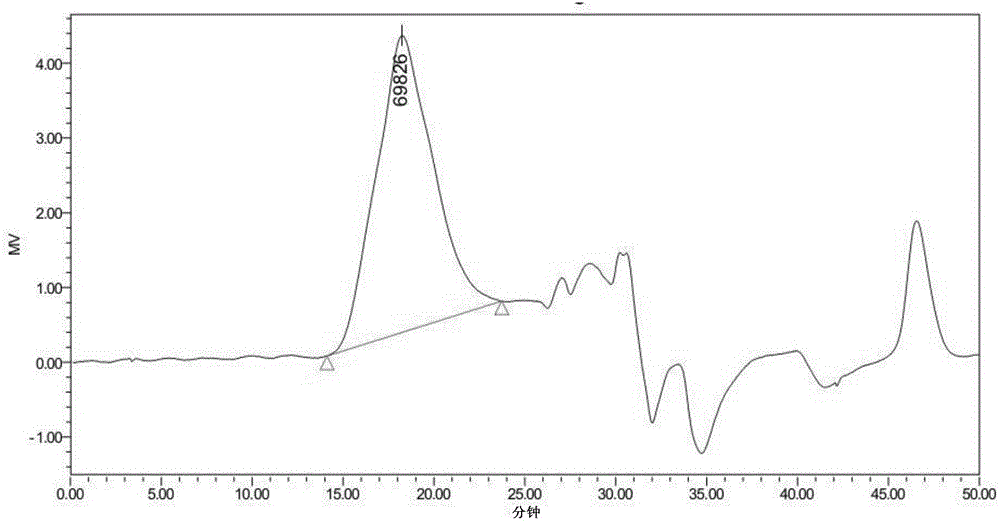
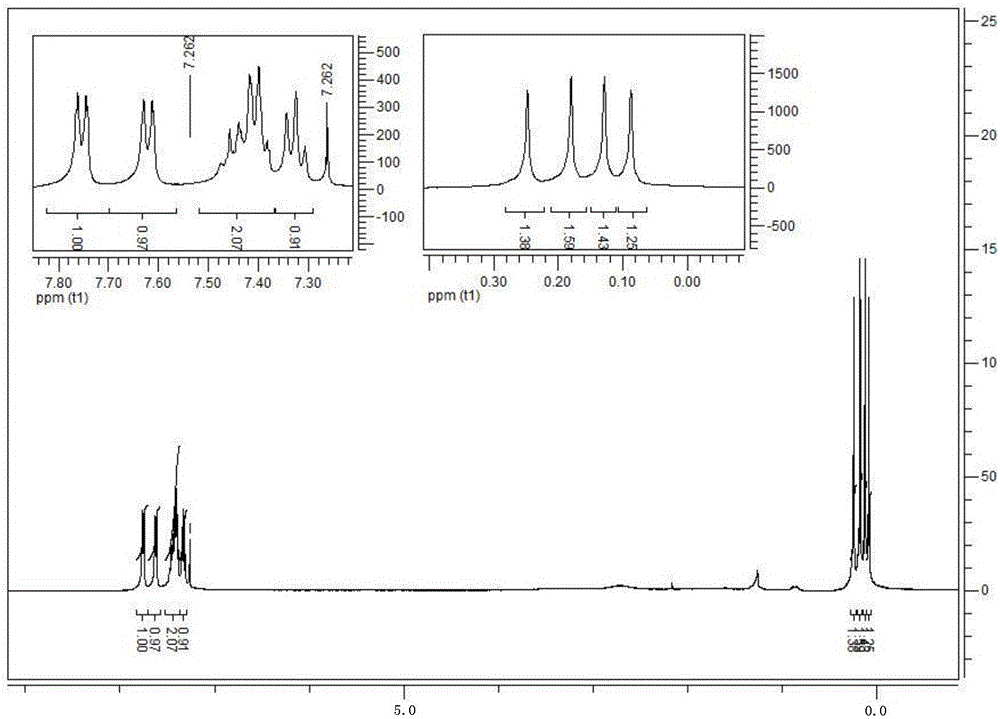
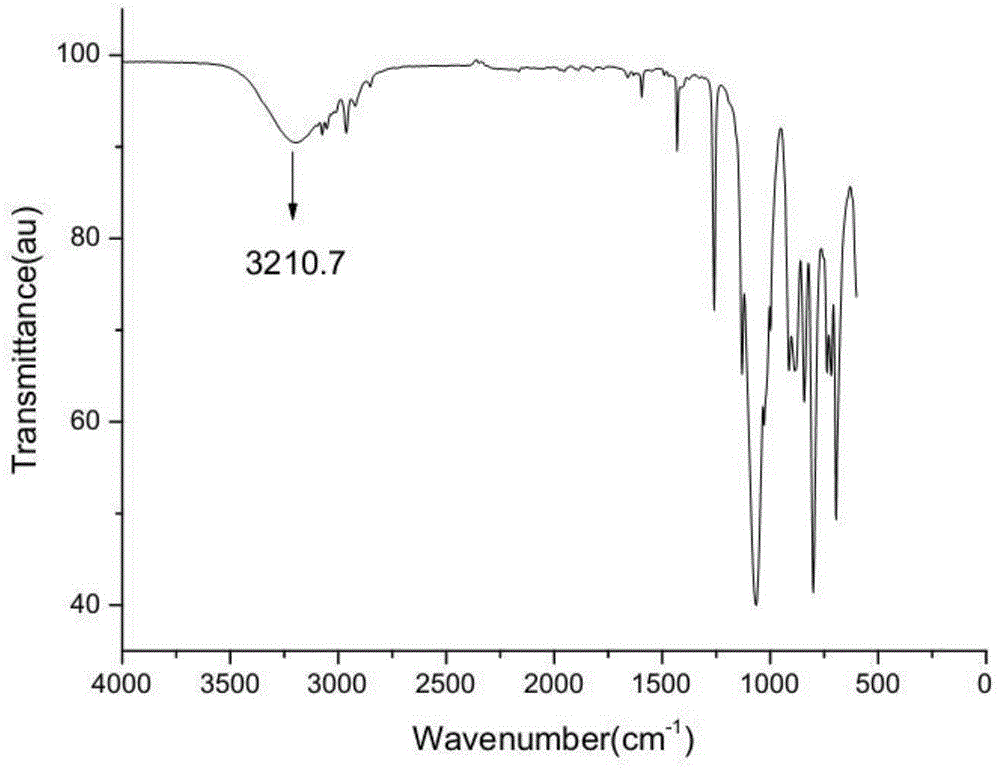
[0050] The gel permeation chromatogram of hyperbranched polysiloxane containing macrocyclic structure with side groups of phenyl and methyl is as follows: figure 1 As shown, the infrared spectrum is shown in figure 2 As shown, the H NMR spectrum is shown as image 3 As shown, the NMR silicon spectrum is shown as Figure 4 As shown, the thermogravimetric spectrum is shown as Figure 5 sh...
Embodiment 2
[0052] Preparation of hyperbranched polysiloxane with methyl side group containing macrocyclic structure
[0053] -60°C, add 10 grams of 1,1,3,3,5,7-hexamethylcyclotetrasiloxane and 10 grams of water into a 100 ml two-necked flask containing 1 gram of nickel acetylacetonate and 50 ml of acetone, Stir for 24 hours. A chromatographic column filled with diatomaceous earth was used to remove nickel acetylacetonate, then the reaction solution was poured into 200 milliliters of methanol, solids were precipitated, filtered to obtain solids, and finally vacuum-dried to obtain white solids with a yield of 65% and a degree of branching of 70 %.
Embodiment 3
[0055] Preparation of macrocyclic hyperbranched polysiloxane with side groups cyclohexyl and vinyl
[0056] At 140°C, 100 grams of 1,3,3,5,7,7-hexacyclohexyl cyclotetrasiloxane, 100 grams of octavinyl cyclotetrasiloxane and 50 grams of water were added to 6 grams of ferrocene and 300 ml of cyclohexanone in a 500 ml two-neck flask, stirred for 10 hours. Remove ferrocene with a chromatographic column filled with aluminum oxide, then pour the reaction solution into 1000 milliliters of methanol, solids are precipitated, filtered to obtain solids, and finally vacuum-dried to obtain white solids, with a yield of 75% and a degree of branching of 80 %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com