Method for synthesizing cyclohexanone-oxime and caprolactam
A technology for caprolactam and cyclohexanone oxime, which is applied in the field of synthesizing cyclohexanone oxime and caprolactam, can solve the problems of many side reactions, low utilization rate of raw materials, difficult operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
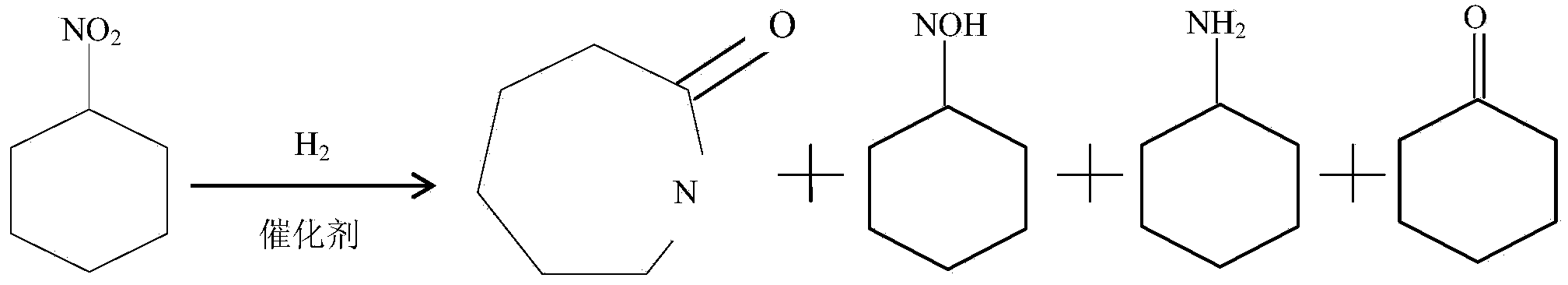
[0033] The catalyst used is Pd / H-ZSM-5. The composition of the catalyst is: Pd=5%, and the rest is carrier H-ZSM-5. 0.3 g of the catalyst was pre-activated with hydrogen, and the activation conditions were as follows: the flow rate of hydrogen gas was 40 mL / min, normal pressure, the heating rate was 10 °C / min, 250 °C, and the reduction time was 3 h. Choose a fixed bed reactor. Add the raw material nitrocyclohexane, the reaction temperature is 350°C, the hydrogen pressure is 0.1MPa, the flow rate of the raw material is 40mL / min, and the liquid space velocity is 0.3h -1 The reaction time was 72h under certain conditions, and samples were taken for analysis. The liquid product was analyzed by gas chromatography. In the product, nitrocyclohexane was 25.66%, caprolactam was 53.65%, cyclohexanone oxime was 9.33%, and cyclohexylamine was 11.36%.
Embodiment 2
[0035] The synthesis reaction was carried out in the same manner as in Example 1, except that the raw material was replaced with a mixed solution of nitrocyclohexane and methanol at a volume ratio of 1:10. Through gas chromatography analysis, the nitrocyclohexane in the product is 22.24%, the caprolactam is 10.77%, the cyclohexanone oxime is 8.70%, and the cyclohexylamine is 58.19%.
Embodiment 3
[0037] In addition to changing the liquid space velocity to 3h -1 Except, carry out the synthetic reaction according to the same method as in Example 2, and analyze by gas chromatography, the nitrocyclohexane in the product is 25%, the caprolactam is 0.14%, the cyclohexanone oxime is 58.54%, and the cyclohexanone is 0.49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com