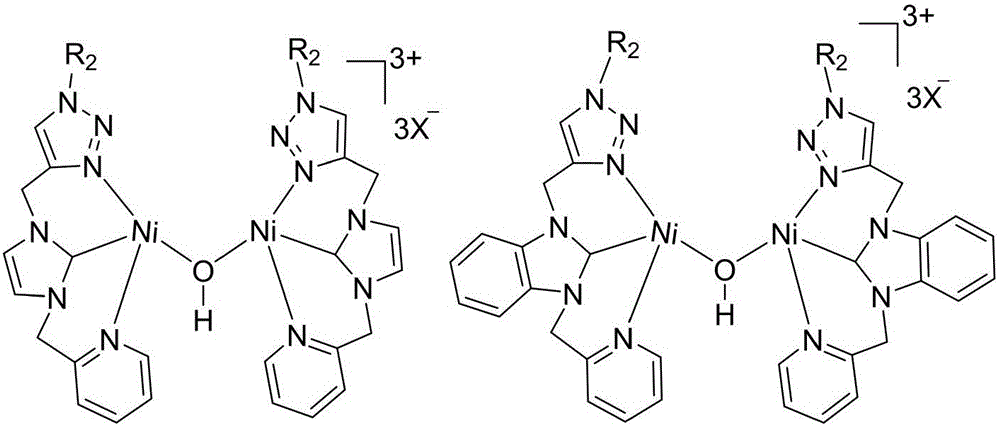
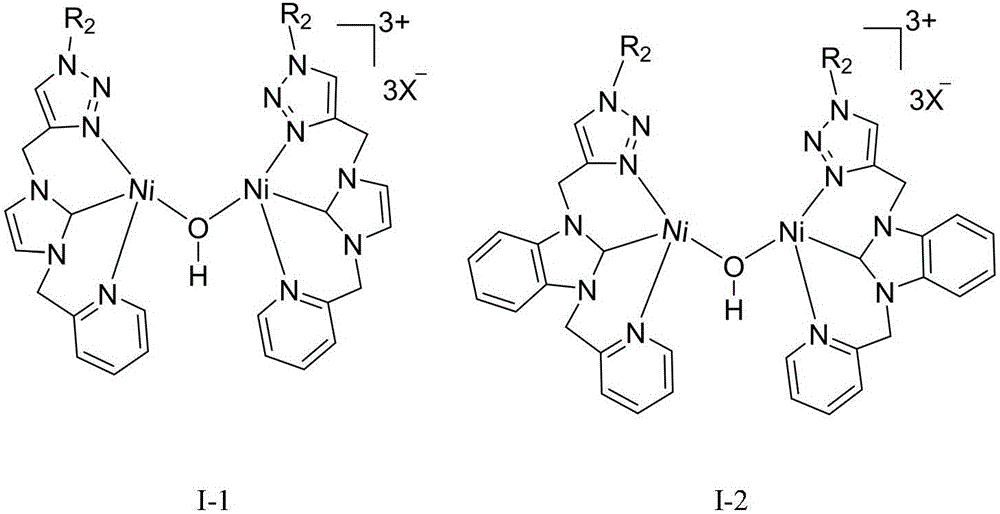
A 1,2,3-triazole functionalized nitrogen-heterocyclic carbene binuclear nickel compound and its preparation method
A nitrogen-heterocyclic carbene and nickel compound technology, applied in the field of organometallic chemistry, can solve the problems of less research on binuclear nickel compounds, and achieve the effects of novel structure, easy preparation, and strong electron donating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] At 50°C, add 1,2,3-triazole functionalized nitrogen heterocyclic carbene ligand L1 (477 mg, 1 mmol), silver oxide (116 mg, 0.5 mmol) and 20 mL of acetonitrile into a Schlenk reaction tube for 10 hours, then add NiCl 2 (PPh 3 ) 2 (654mg, 1mmol), stirred and reacted at 25°C for 6 hours, centrifuged to filter out the precipitate, concentrated the filtrate to 2mL, added 20mL of anhydrous ether, precipitated a solid, collected the solid by filtration, and vacuum-dried at 30°C for 10h to obtain the molecular structure: The 1,2,3-triazole functionalized azacyclic carbene binuclear nickel compound of 1 was 700 mg, and the yield was 55%. 1 H NMR (400MHz, d 6 -DMSO): δ8.54(b, 1H), 8.30(s, 1H), 7.89(t, J=7.2Hz, 1H), 7.79(s, 2H), 7.48(d, J=7.6Hz, 1H) ,7.39-7.33(m,6H),5.64(s,2H),5.68(s,4H)ppm. 13 C NMR (400MHz, d 6 -DMSO): δ156.9 (Ni-C), 153.9, 150.1, 141.2, 138.0, 137.5, 136.2, 129.3, 128.8, 125.1, 124.2, 123.9, 123.1, 123.0, 53.6, 53.5, 44.2ppm.
[0043] Utilizi...
Embodiment 2
[0047]
[0048] At 50°C, add 1,2,3-triazole functionalized nitrogen heterocyclic carbene ligand L2 (527mg, 1mmol), silver oxide (116mg, 0.5mmol) and 20mL of acetonitrile into the Schlenk reaction tube, react for 15 hours, then add NiCl 2 (PPh 3 ) 2 (654mg, 1mmol), stirred and reacted at 25°C for 7 hours, centrifuged to filter out the precipitate, concentrated the filtrate to 2mL, added 20mL of anhydrous ether, precipitated a solid, collected the solid by filtration, and vacuum dried at 30°C for 10h to obtain the molecular structure formula: The 1,2,3-triazole functionalized azacyclic carbene binuclear nickel compound of 2 was 714 mg, and the yield was 52%. 1 H NMR (400MHz, d 6 -DMSO): δ8.79(b,1H),8.46-8.44(m,1H),8.39(s,1H),8.29(t,J=8.0Hz,1H),8.18-8.16(m,1H), 8.06(d,J=8.0Hz,1H),7.78-7.72(m,3H),7.37-7.30(m,6H),5.98(s,2H),5.68(s,2H),5.63(s,2H) ppm. 13 CNMR (400MHz, d 6 -DMSO): δ157.9 (Ni-C), 153.9, 149.9, 147.6, 143.1, 141.1, 140.5, 136.2, 131.7, 130.1, 129.3, 128.8, 12...
Embodiment 3
[0053]
[0054] At 50°C, add 1,2,3-triazole functionalized nitrogen heterocyclic carbene ligand L3 (443mg, 1mmol), silver oxide (116mg, 0.5mmol) and 20mL of acetonitrile into the Schlenk reaction tube, react for 15 hours, then add NiCl 2 (PPh 3 ) 2 (654mg, 1mmol), stirred and reacted at 25°C for 8 hours, centrifuged to filter out the precipitate, concentrated the filtrate to 2mL, added 20mL of anhydrous ether, precipitated a solid, collected the solid by filtration, and vacuum dried at 30°C for 10h to obtain the molecular structure formula: The 1,2,3-triazole functionalized azacyclic carbene binuclear nickel compound of 3 was 747 mg, and the yield was 62%. 1 H NMR (400MHz, DMSO-d 6 ):δ9.05(s,1H,triazole),8.63(d,1H),8.16-8.14(m,2H),7.87(s,1H),7.82(s,1H),7.61-7.58(m,1H ),5.68(s,2H),5.65(s,2H),4.18(t,J=7.8Hz,2H),1.79-1.74(m,2H),1.31-1.24(m,2H),0.90(t, 3H,J=7.2Hz,). 13 C NMR (100MHz, DMSO-d 6 ): δ154.2 (Ni-C), 149.5, 148.7, 142.0, 136.8, 125.1, 123.2, 123.1, 122.5, 114.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com