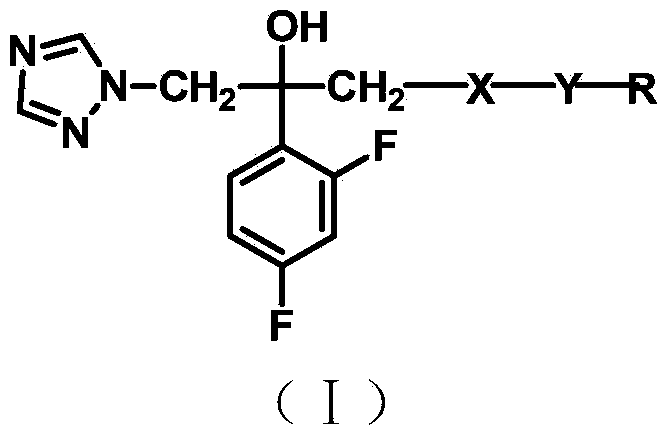
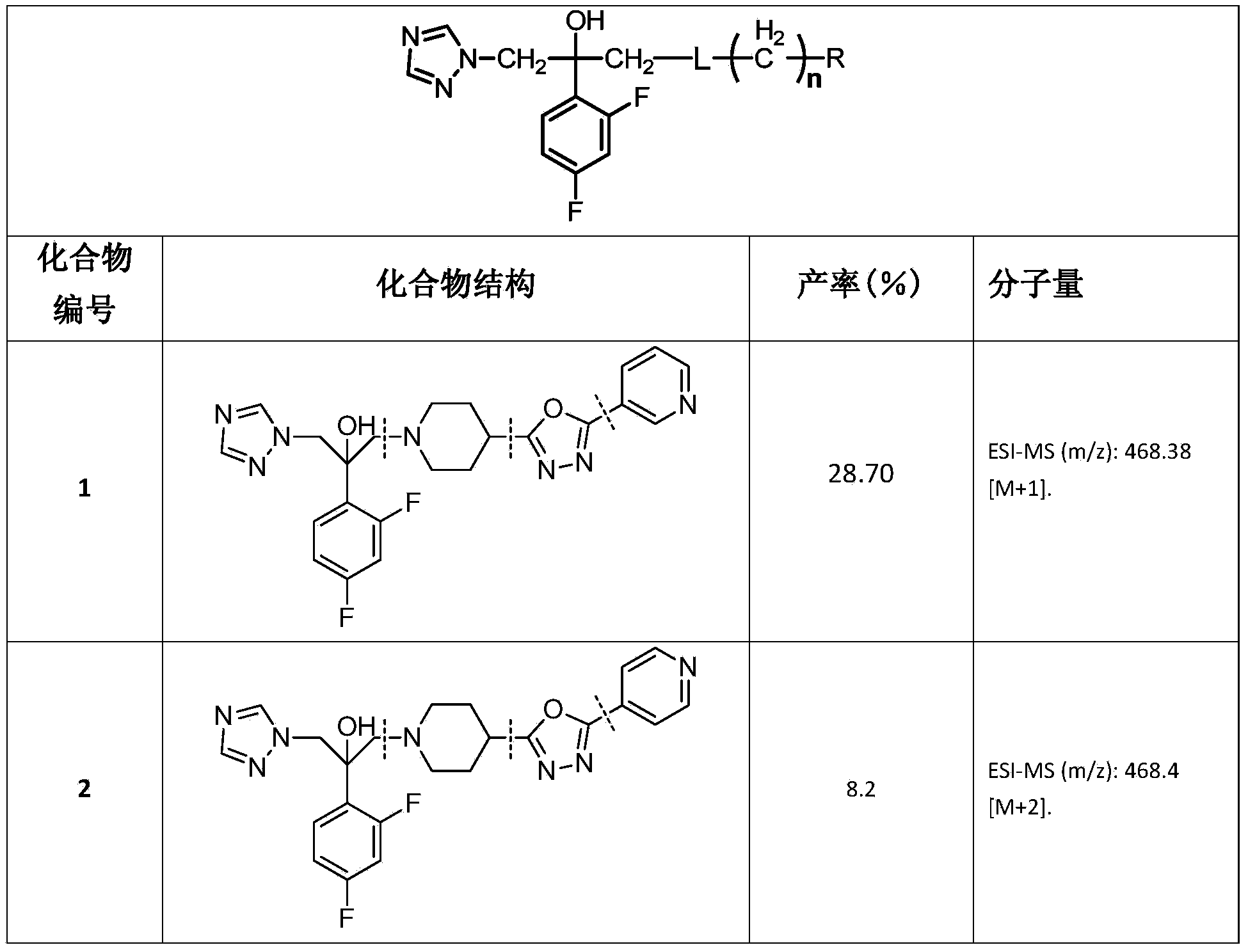
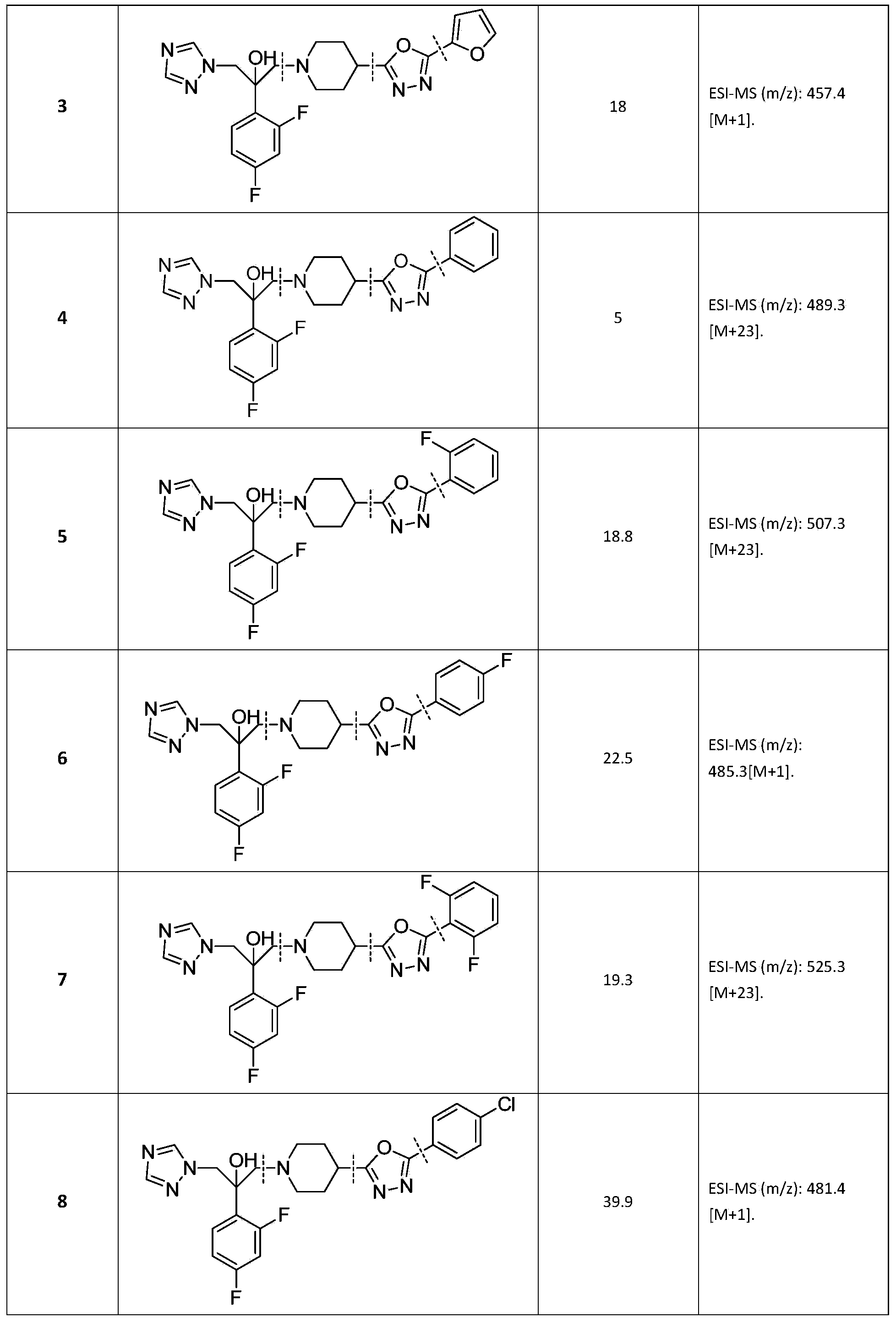
Triazole alcohol antifungal compound with side chain containing piperidine oxadiazole as well as preparation method and application thereof
A technology of oxadiazoles and compounds, applied in the application of medicines, triazole alcohol antifungal compounds and their preparation fields, can solve the problems of large side effects, narrow antibacterial spectrum, and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Preparation of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole mesylate (Ⅱ)
[0101] Take 2-(1H-1,2,4-triazol-1-yl)-2',4'-difluoroacetophenone (Ⅰ) 29.8g (0.115mol), trimethyl iodine oxysulfide 25.3g ( 0.115mol), 1.6g of trimethylhexadecylammonium bromide, put into a 500ml three-necked flask, add 180ml of toluene and 225ml of 20% sodium hydroxide solution (w / w), heat at 60°C for 3 hours, and the reaction ends Finally, separate the toluene layer, extract the water layer with ethyl acetate (100mlX2), combine the organic layers, wash with water until neutral, evaporate the solvent to dryness, add 120ml of ethyl acetate to the residual solution and dilute with 8.3g of formazan at 0°C. 2ml of ethyl acetate of sulfonic acid precipitated a pale yellow solid, which was filtered and recrystallized from methanol to obtain 21.71g of compound IV with a yield of 56.7% and a melting point of 128-129°C.
Embodiment 2
[0102] Embodiment 2: Preparation of N-Boc-4-hydrazinecarbonylpiperidine (III)
[0103] 2 g (7.77 mmol) of methyl N-tert-butoxycarbonyl-4-piperidinecarboxylate was dissolved in a (1:1) mixed solvent (30 mL) of hydrazine hydrate and ethanol, and stirred overnight at room temperature. Ethanol was concentrated to dryness, added to water (50 mL), dried with dichloromethane (50 mL×3), anhydrous sodium sulfate, filtered, and concentrated to obtain 1.8 g of white solid with a yield of 95%.
Embodiment 3
[0104] Embodiment 3: Preparation of N-Boc-4-hydrazide carbonyl piperidine compound (IV class compound)
[0105] Take 0.2g (0.82mmol) of N-Boc-4-hydrazinecarbonylpiperidine, 0.12g (1.23mmol) of triethylamine and dissolve it in dichloromethane (20mL), and slowly add 0.22g (1.23mmol) of nicotinyl chloride under ice bath ), and stirred at room temperature for 2 hours. Add dichloromethane (30mL) to dilute, wash with saturated brine (20mL×3), dry over anhydrous sodium sulfate, filter, concentrate, and obtain crude product by silica gel column chromatography (ethyl acetate:methanol=50:1-30: 1), 0.2 g of white solid was obtained, and the yield was 70%.
[0106] Other IV compounds are prepared by using III as a raw material, reacting with different aryl formyl chlorides as raw materials, and repeating the steps in Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com