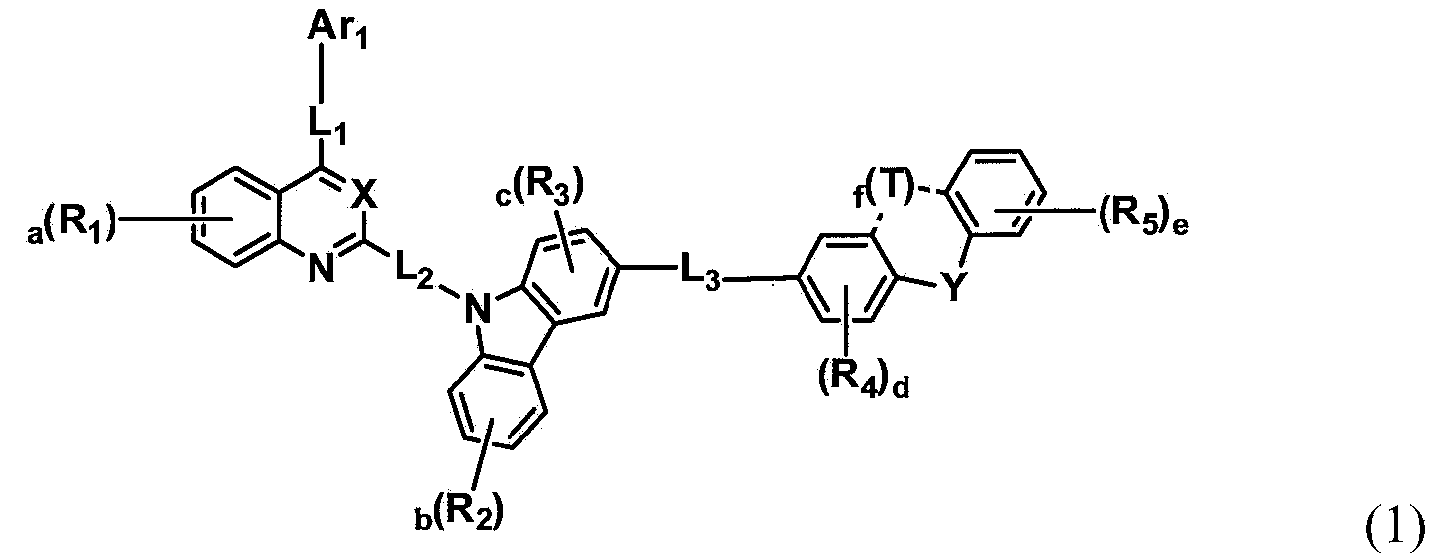
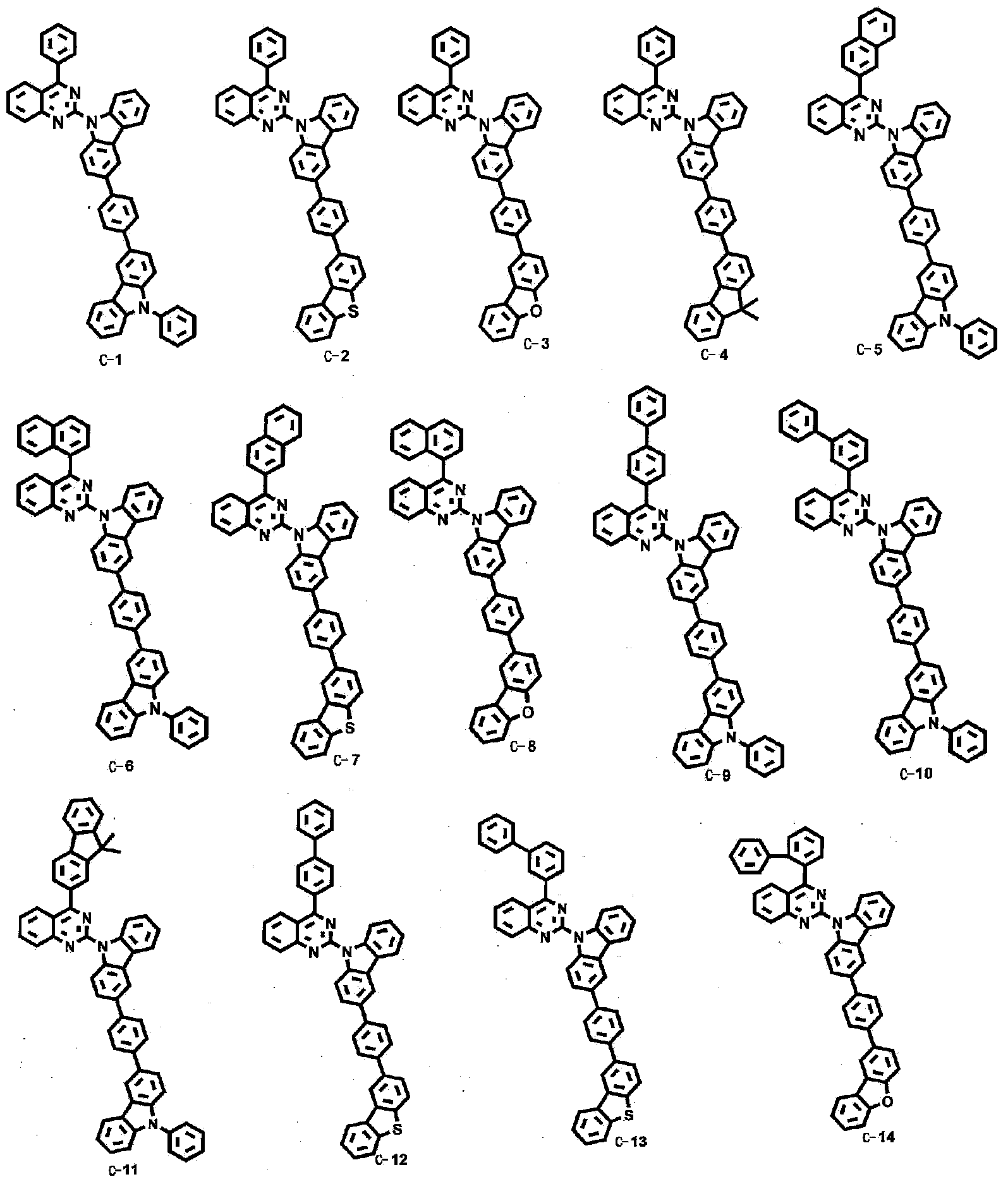
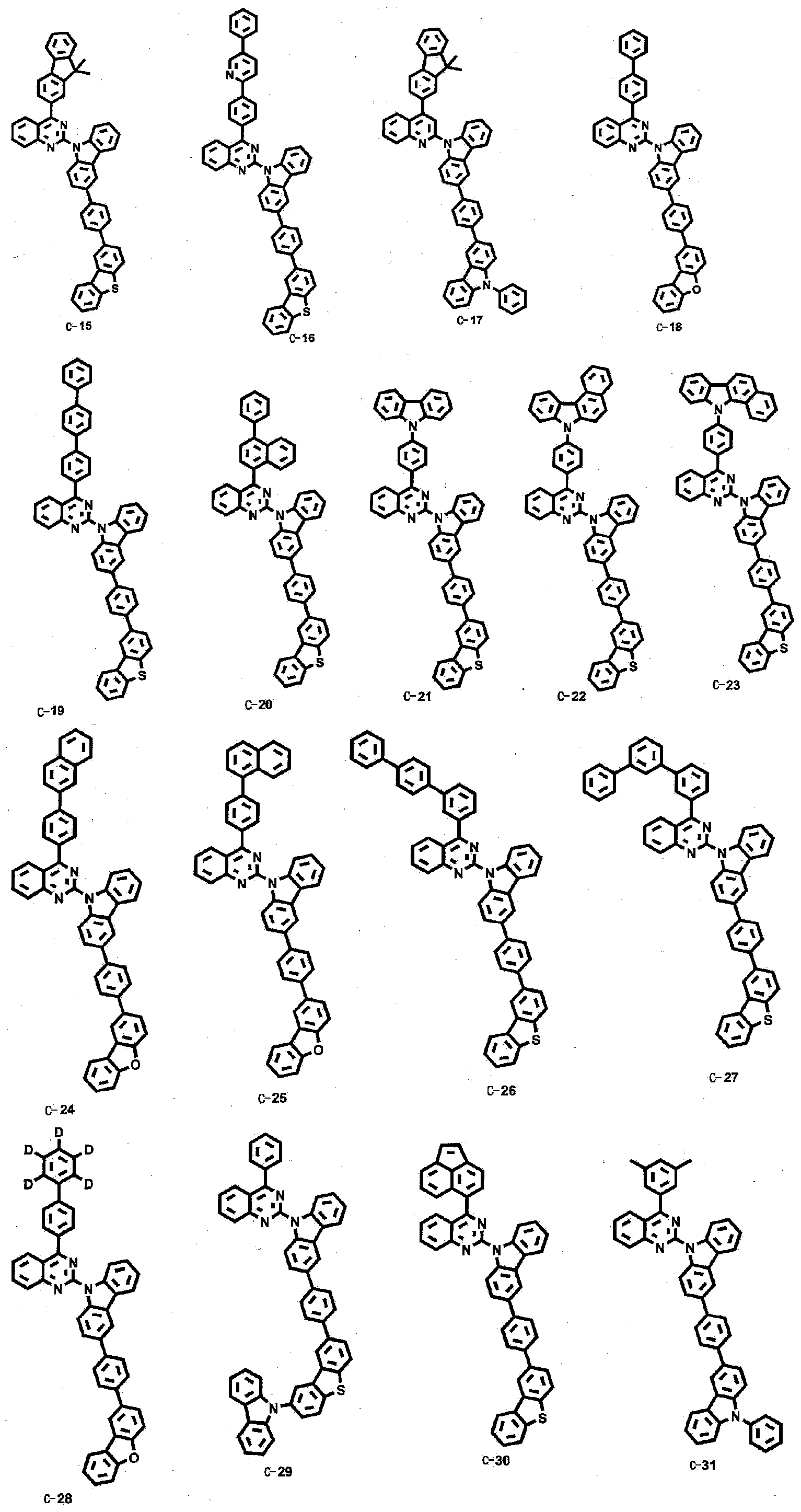
Novel organic electroluminescent compounds and organic electroluminescent device comprising the same
A compound, electroluminescence technology, applied in electroluminescence light sources, organic chemistry, electro-solid devices, etc., can solve the problems of degradation, no advantage in power efficiency, low glass transition temperature, etc., and achieve the effect of long working life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: the preparation of compound C-1
[0077]
[0078] Preparation of compound C-1-1
[0079] To a 2L round bottom flask (RBF) was added N-phenylcarbazole-3-boronic acid (30.0 g, 105.0 mmol), 4-bromoiodobenzene (44.0 g, 157.0 mmol), tetrakis(triphenylphosphine)palladium ( O)[Pd(PPh 3 ) 4 ] (3.5g, 3.14mmol), Na 2 CO 3 (33.0 g, 313.0 mmol), toluene (600 mL), ethanol (EtOH) (150 mL) and distilled water (150 mL), the reaction mixture was stirred at 110° C. for 2 hours. Use ethyl acetate (EA) / H 2 O to work up the reaction mixture with MgSO 4 Dry to remove moisture, distill under reduced pressure and separate by column chromatography using dichloromethane (MC) and hexane to obtain compound C-1-1 (30.0 g, 72%) as a yellow solid.
[0080] Preparation of compound C-1-2
[0081] After compound C-1-1 (30.0 g, 75.3 mmol) was added to 1L RBF and replaced with nitrogen, tetrahydrofuran (THF) (400.0 mL) was added to the flask. The solution was cooled to -78°C...
Embodiment 2
[0086] Embodiment 2: the preparation of compound C-76
[0087]
[0088] Preparation of Compound C-76-1
[0089] Add 4-(diphenylamino)phenylboronic acid (14.0g, 48.4mmol), 3-bromocarbazole (10.0g, 40.3mmol), Pd(PPh 3 ) 4 (2.4g, 2.0mmol), K 2 CO 3 (13.0 g, 96.8 mmol), toluene (200 mL), EtOH (50 mL) and distilled water (50 mL), the reaction mixture was stirred at 110 °C for 24 hours. Use EA / H 2 The reaction mixture was extracted with MgSO 4 Dry to remove moisture, distill under reduced pressure and separate by column chromatography using MC and hexane to obtain compound C-76-1 (14.0 g, 84%) as a yellow solid.
[0090] Preparation of compound C-76
[0091] Compound C-76-1 (6.0 g, 14.6 mmol) and DMF (75 mL) were added to 250 mL RBF, and stirred to dissolve. NaH (0.9 g, 60% dispersion in mineral oil, 21.9 mmol) was added to the mixture and the reaction mixture was stirred for 30 minutes. 2-Chloro-4-phenylquinazoline (4.0 g, 17.5 mmol) was slowly added to the reacti...
Embodiment 3
[0092] Embodiment 3: the preparation of compound C-77
[0093]
[0094] Compound C-76-1 (4.0 g, 10.2 mmol) and DMF (50 mL) were added to 250 mL RBF, and stirred to dissolve. NaH (0.6 g, 60% dispersion in mineral oil, 15.4 mmol) was added to the mixture, and the reaction mixture was stirred for 30 minutes. 4-([1,1'-biphenyl]-3-yl)-2-chloroquinazoline (4.0 g, 12.3 mmol) was slowly added to the reaction mixture. After the addition, the reaction mixture was stirred at 50 °C for 2 hours. The reaction mixture was quenched with methanol and filtered to obtain a solid. The resulting solid was dried in a vacuum oven and separated by column chromatography using MC and hexane to give compound C-77 (2.1 g, 30%) as a yellow solid.
[0095] The physical properties of the compounds of the present invention prepared in Examples 1-3 are listed in Table 1 below:
[0096] Table 1
[0097]
[0098] Device Example 1: Manufacturing OLED devices using the organic electroluminescent co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com