Spirobenzanthrone derivative and electronic device
A technology for spirobenzoxanthrone and electronic devices, which is applied in the field of spirobenzoxanthrone derivatives, electronic devices, and electronic devices of spirobenzoxanthrone derivatives, and achieves simple preparation method, easy-to-obtain raw materials, and luminescence. Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Embodiment 1: the synthesis of compound 3
[0171] (Synthesis of Intermediate 1-1)
[0172] The synthetic route of intermediate 1-1 is as follows:
[0173]
[0174] Under nitrogen protection, the intermediate acridone (820 mg, 4.2 mmol), N-([1,1'-biphenyl]-4-yl)-N-(4-bromophenyl )-[1,1'-biphenyl]-4-amine (2.4g, 5mmol), palladium acetate (18mg, 0.08mmol), tri-tert-butylphosphine tetrafluoroborate (73mg, 0.25mmol), tert Sodium butoxide (806mg, 8.4mmol) and 120mL of toluene were stirred under reflux for 12 hours. After the reaction is complete, evaporate the solvent, dissolve the residue with 200 mL of dichloromethane and 50 mL of water, wash with water, separate the organic layer, extract the aqueous layer twice with 15 mL of dichloromethane, combine the organic layers, evaporate the solvent, and pass the residue through the column After chromatographic separation (petroleum ether:dichloromethane=1:1 (V / V)), the solvent was evaporated and dried to obtain 1.4 g of li...
Embodiment 2
[0180] Embodiment 2: the synthesis of compound 98
[0181] (Synthesis of Intermediate 1-2)
[0182] The synthetic route of intermediate 1-2 is shown below:
[0183]
[0184]Under the protection of nitrogen, 2.9 g (8.9 mmol) of 2-bromotriphenylamine and 150 mL of anhydrous tetrahydrofuran were added to a dry and clean 250 mL three-necked flask, and stirred and dissolved at room temperature. The system was cooled to -78°C, and 3.9 mL (2.5 M, 9.8 mmol) of n-butyllithium was added dropwise at this temperature, and stirring was continued at this temperature for 1.5 h after the addition was complete. Subsequently, 2.5 g (8.1 mmol) of 9-bromobenzanthrone was added in one batch. After the addition, the cold bath was removed, and the reaction returned to room temperature by itself and continued to stir overnight. After the reaction, it was washed with water, dried, and spin-dried to obtain a white solid.
[0185] The above white solid was transferred to a 250mL one-necked bottle ...
Embodiment 3
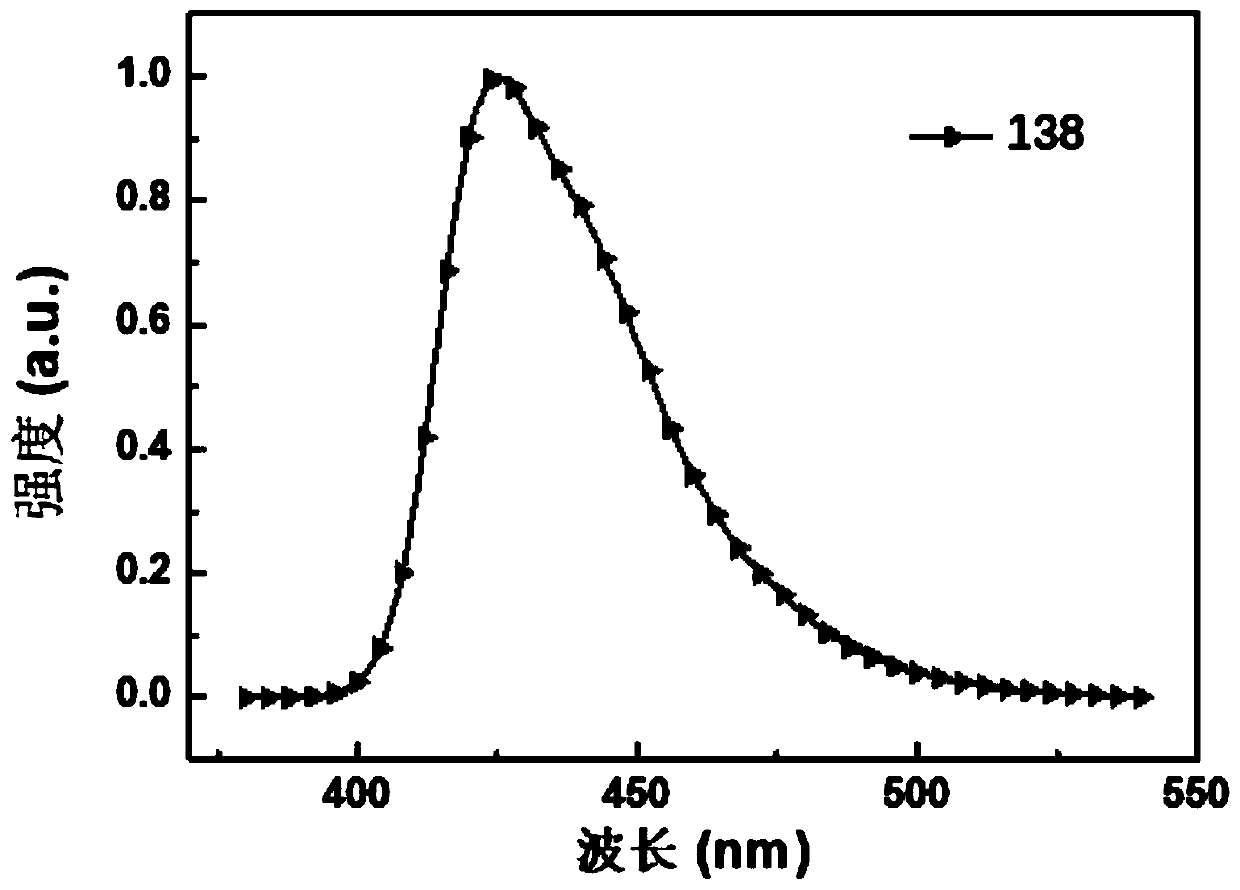
[0190] Embodiment 3: the synthesis of compound 138
[0191] (Synthesis of Intermediates 1-3)
[0192] The synthetic routes of intermediates 1-3 are shown below:
[0193]
[0194] Under the protection of nitrogen, 2.9 g (8.9 mmol) of 2-bromotriphenylamine and 150 mL of anhydrous tetrahydrofuran were added to a dry and clean 250 mL three-necked flask, and stirred and dissolved at room temperature. The system was cooled to -78°C, and 3.9 mL (2.5 M, 9.8 mmol) of n-butyllithium was added dropwise at this temperature, and stirring was continued at this temperature for 1.5 h after the addition was completed. Subsequently, 2.5 g (8.1 mmol) of 3-bromobenzanthrone was added in one batch. After the addition, the cold bath was removed, and the reaction was warmed to room temperature by itself and continued to stir overnight. After the reaction, it was washed with water, dried, and spin-dried to obtain a white solid.
[0195] The above white solid was transferred to a 250mL one-necke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com