Fiber-enhanced drug-loading hydrogel keratoprosthesis skirt stent and preparation method thereof
An artificial cornea and fiber-reinforced technology, applied in medical science, prosthesis, etc., can solve the problems of long biological fixation process of artificial cornea, slow corneal fibers and blood vessels, and complications in the eye, and achieve excellent biocompatibility and safety. Permeability, good biomimeticity, the effect of reducing the chance of complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
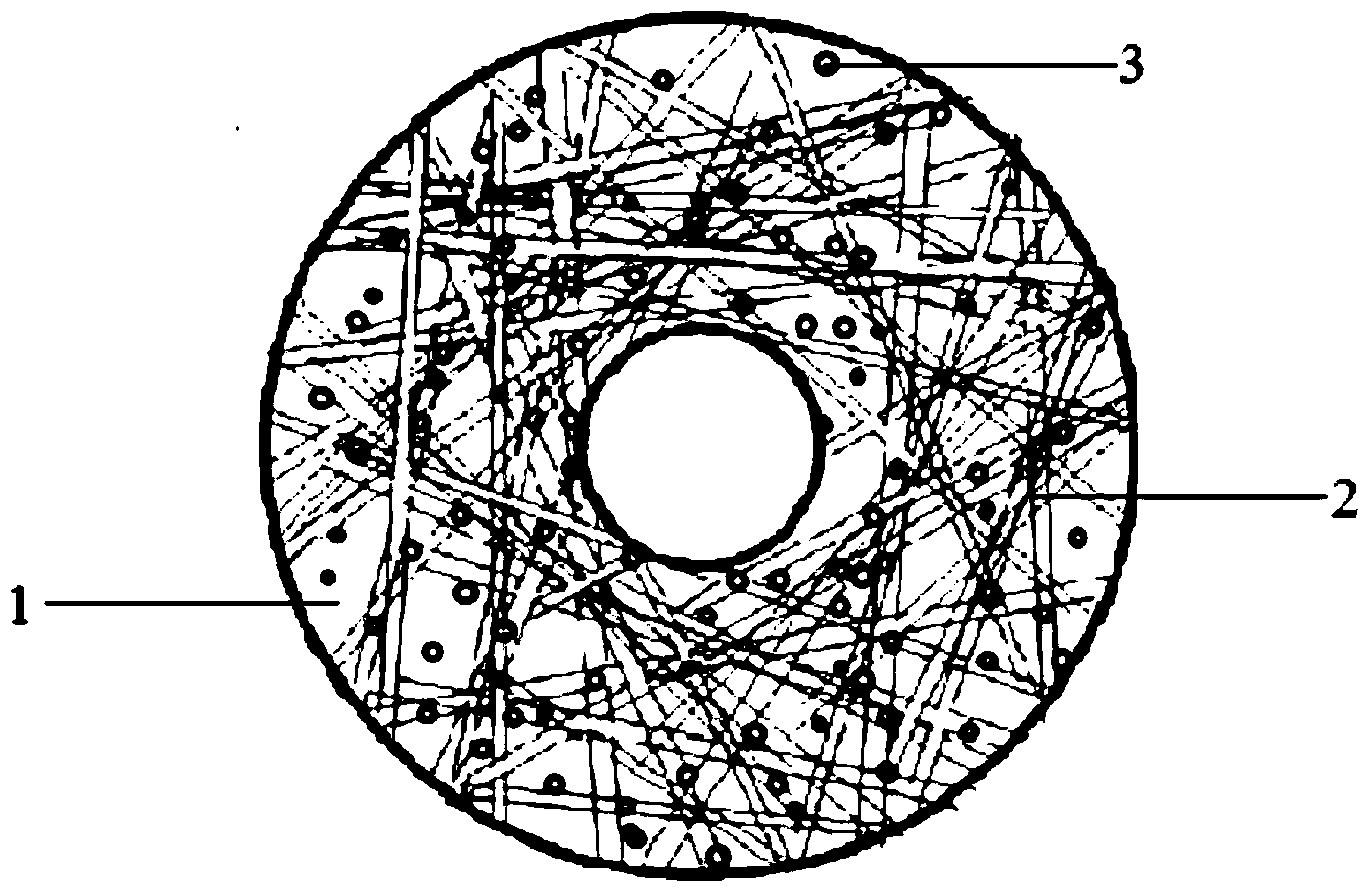
[0027] In this embodiment, the fiber-reinforced drug-loaded hydrogel artificial corneal skirt support is a sheet-shaped ring, and its structural diagram is shown in figure 1 , its preparation method is as follows:
[0028] (1) Prepare biodegradable drug-loaded microspheres by emulsification and solidification method: add 1g of Span-80, 0.6g of Tween-80, and 46g of liquid paraffin into the container and stir until the mixture is uniform to obtain a mixed solution, and add 10g of the mixed aqueous solution of gelatin and αFGF, in the mixed aqueous solution of gelatin and αFGF, the quality of gelatin is 1.5g, the quality of αFGF is 0.2g, stirs to form emulsion, and in the container, add the glutaraldehyde 2.4mL that concentration is 50wt% , continue to stir for 30 minutes, the emulsion droplets are cross-linked and solidified into balls during the stirring process, then add petroleum ether equal to the volume of the mixed solution and stir for 15 minutes to break the emulsion, ce...
Embodiment 2
[0034] In this embodiment, the structural schematic diagram of the fiber-reinforced drug-loaded hydrogel artificial corneal skirt stent is shown in figure 1 , its preparation method is as follows:
[0035] (1) Prepare biodegradable drug-loaded microspheres by emulsification and solidification method: add 1g of Span-80, 0.6g of Tween-80, and 46g of liquid paraffin into the container and stir until the mixture is uniform to obtain a mixed solution, and add 10g of a mixed aqueous solution of sodium alginate and βFGF. In the mixed aqueous solution of sodium alginate and βFGF, the quality of sodium alginate is 1.6g, and the quality of αFGF is 0.2g. Then stir to form an emulsion, and add a concentration of 50wt to the container. % glutaraldehyde 2.4mL, continue to stir for 30min, the emulsion droplets are cross-linked and solidified into balls during the stirring process, then add petroleum ether equal to the volume of the mixed solution and stir for 15min to break the emulsion, aft...
Embodiment 3
[0040] In this embodiment, the structural schematic diagram of the fiber-reinforced drug-loaded hydrogel artificial corneal skirt stent is shown in figure 1 , its preparation method is as follows:
[0041] (1) Prepare biodegradable drug-loaded microspheres by emulsification and solidification method: add 1g of Span-80, 0.6g of Tween-80, and 46g of liquid paraffin into the container and stir until the mixture is uniform to obtain a mixed solution, and add The mixed aqueous solution 10g of collagen protein and βFGF, in the mixed aqueous solution of described collagen protein and βFGF, the quality of collagen protein is 1.7g, the quality of βFGF is 0.3g, stirs to form emulsion then, and adding concentration is 50wt% pentamethylene in the container Dialdehyde 2.4mL, continue to stir for 30min, the emulsion droplets are cross-linked and solidified into balls during the stirring process, then add petroleum ether equal to the volume of the mixed solution and stir for 15min to break t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com