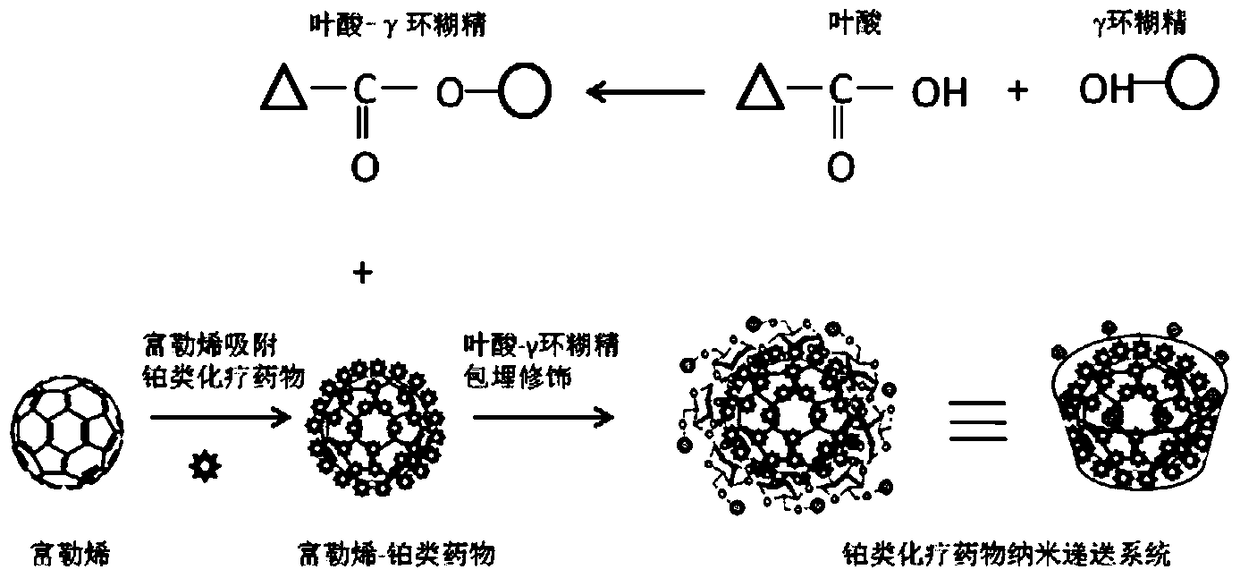
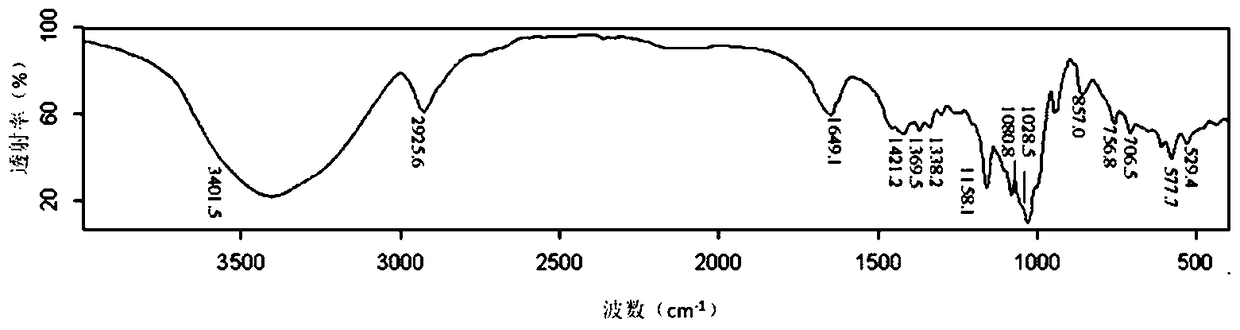
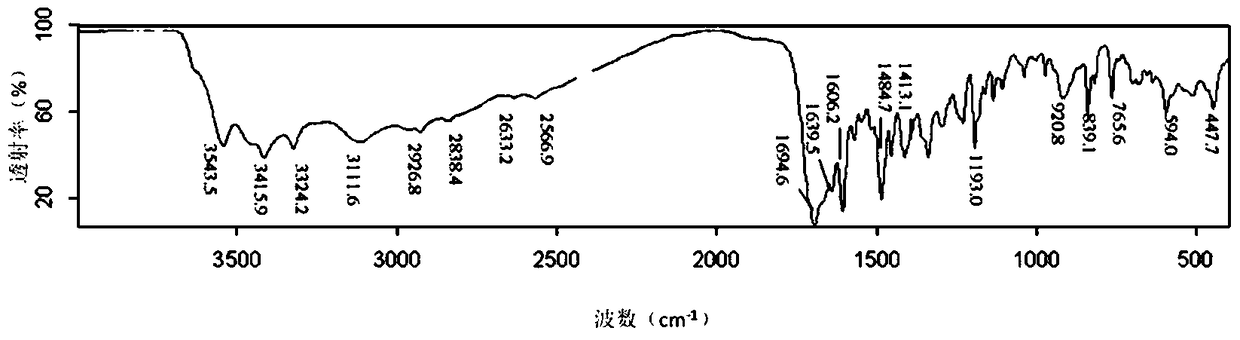
Folic acid-cyclodextrin conjugate, drug delivery carrier, preparation method and use
A technology for delivering carriers and conjugates, applied in the field of medicine and chemical industry, can solve the problems of neurotoxicity, inability to achieve therapeutic effects, poor targeting, etc., and achieve high hydrophilicity, good external hydrophilicity, and improved targeting. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0059] (1) Raw materials:
[0060] Fullerene C 60 : Purchased from Puyang Yongxin Fullerene Technology Co., Ltd., purity ≥ 98%.
[0061] Folic acid: purchased from Sigma, purity ≥ 97%, Lot: 081M1546V.
[0062] γ-cyclodextrin: purchased from Adamas Reagent Company, purity ≥ 98%, Lot: P1064982.
[0063] Carboplatin: purchased from Beijing Ouhe Technology Co., Ltd., purity ≥ 98%, batch number: 120330.
[0064] 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride: purchased from Adamas Reagent Company, purity ≥ 97%, Lot: P1064982.
[0065] N-Hydroxysuccinimide: purchased from Sinopharm Chemical Reagent Co., Ltd., purity ≥ 97%, batch number: F20101019.
[0066] 5% (w / w) glucose injection: purchased from Shijiazhuang Siyao Group, batch number: 110505702.
[0067] (2) Preparation process: the synthetic route of the pharmaceutical composition (drug delivery system) is as follows figure 1 shown.
[0068] a) Synthesis of folic acid-γ-cyclodextrin conjugate: the synthetic r...
preparation example 2
[0076] Grind 1.5 parts by weight of folic acid-γ-cyclodextrin conjugate and 1 part by weight of pre-adsorbed body in methanol (analytically pure, ≥99.5%) in the dark for 3 hours, collect the ground product, dry it with nitrogen, add 20 mL of double-distilled Stir with water, then centrifuge at 5000r / min for 2min to remove residual C 60 Fragments, stored at 4°C protected from light. Others were the same as in Example 1 to obtain drug delivery system 2.
[0077] The present invention includes but not limited to using carboplatin (C 6 h 12 N 2 o 4 Pt) as the active ingredient of the drug, cisplatin (Pt(NH 3 ) 2 Cl 2 ), oxaliplatin (C 8 h 14 N 2 o 4 Pt), nedaplatin (C 2 h 8 N 2 o 3 Pt), Le Platinum (C 9 h 18 N 2 o 3 Pt), Saite Platinum (C 10 h 22 C l2 N 2 o 4 Pt) and platinum prodrugs and other types of pharmaceutical active ingredients can be used to prepare the medicament of the present invention. Wherein, platinum prodrugs include but not limited to glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com