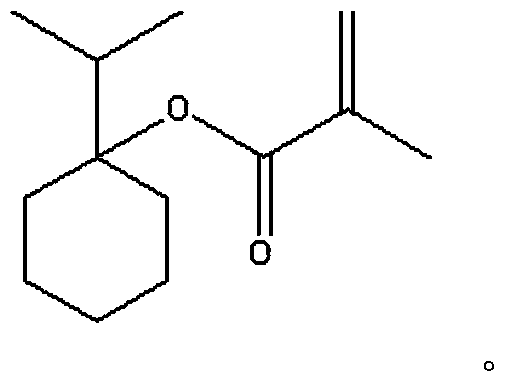
1-isopropylcyclohexanol methyl propionate and preparation method thereof
A technology of alcohol methyl propionate and isopropyl cyclohexanol, which is applied in the field of synthesis of organic compounds, can solve the problems of many side reactions, low reaction yield, and inapplicability to industrial production, so as to improve yield and operate Simple process and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1) The reaction equation is as follows:
[0064]
[0065] (2) Specific process steps:
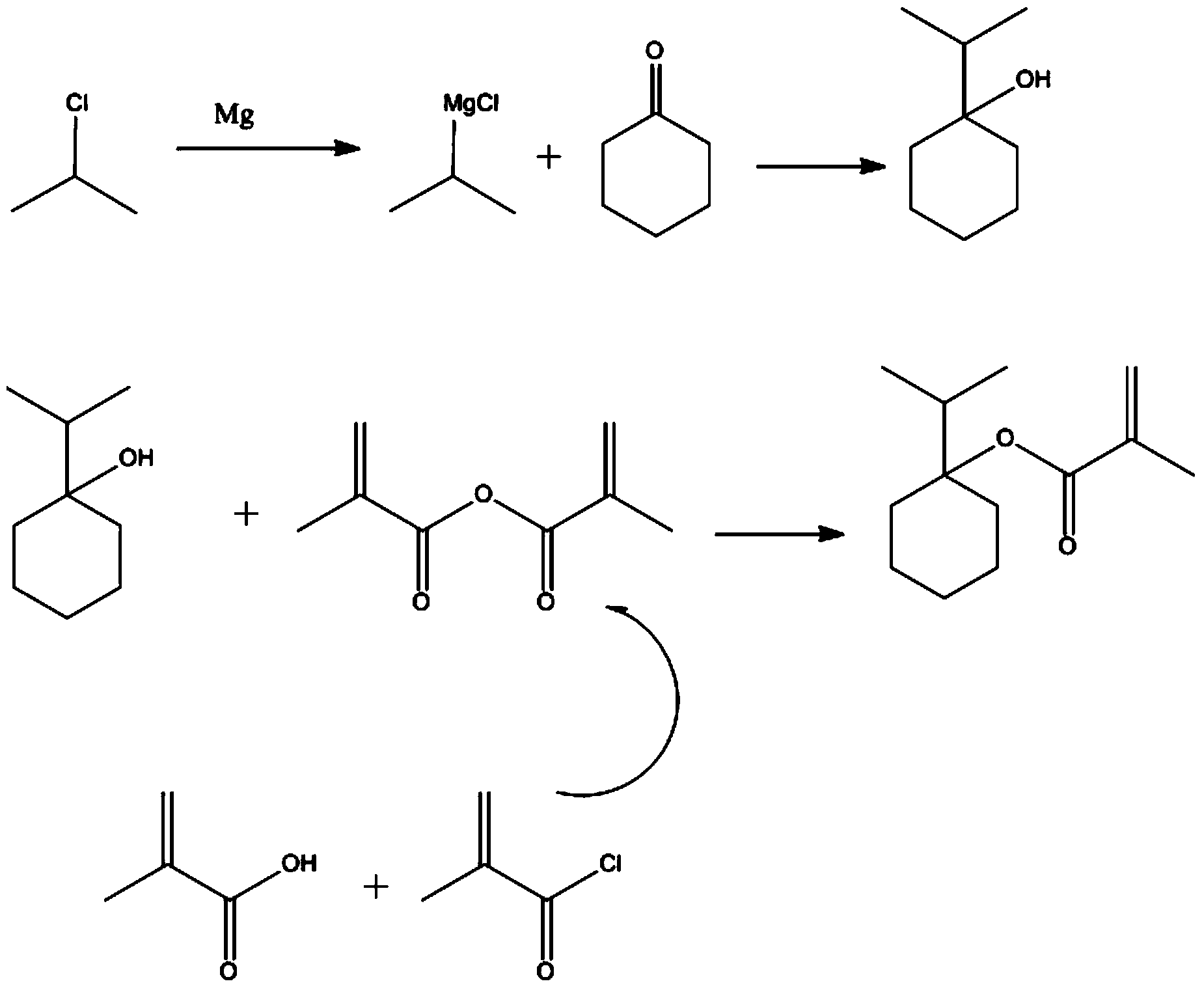
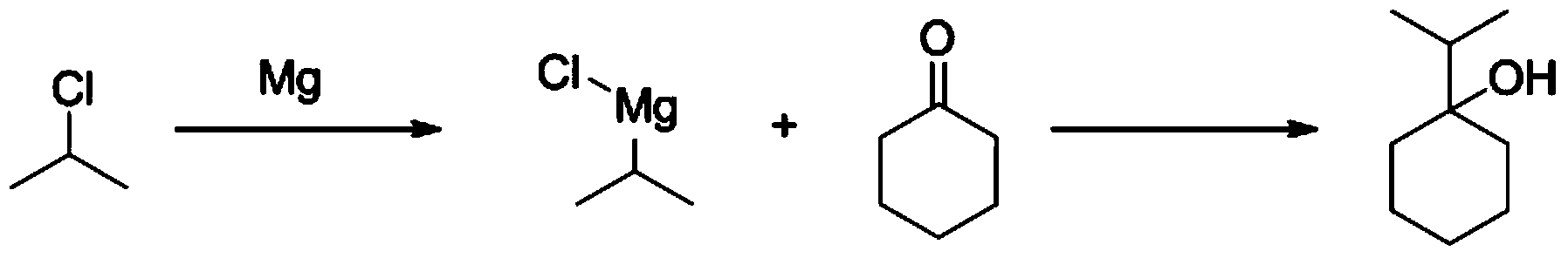
[0066] Under the protection of nitrogen, add 245g of magnesium chips to 738.7g of tetrahydrofuran, add 1ml of 1,2-dibromoethane, and stir well. At room temperature (30 degrees), start to add 2-chloropropane (800g) in tetrahydrofuran (1023.5g) solution, after about 10% dropwise, stop the 2-chloropropane solution, and heat to 60 degrees, the reaction gradually starts, At this time, a large amount of heat is released, and the heat source needs to be removed, and the temperature is changed to a water bath to cool down. After keeping the reaction exothermic stable, continue to add (2-chloropropane) bromoethane solution dropwise to maintain a slight boiling (about 67 degrees), and finish dripping in 1.5 hours After dripping, keep stirring at this temperature for 2 hours (the solution is dark gray at this time).
[0067] Cool the reaction solution to 0-5 degrees, then add a solution of cyclohex...
Embodiment 2
[0074] (1) The reaction equation is as follows:
[0075]
[0076] (2) Specific process steps:
[0077] Add 1250g methacrylic acid and 1518g methacryloyl chloride to 8379g dichloromethane at room temperature, cool to -10-0 degrees, add 1575g triethylamine dropwise, control the internal temperature <0 degrees, about 45 minutes after dripping, take a sample (central control 1). The reaction mixture was poured into 10 kg of ice water, stirred for 30 minutes and then separated into layers. The upper aqueous phase was extracted with dichloromethane (3990g×1), the organic phases were combined, and the organic phase was washed with water (2000g×1) and rotated. Dry solvent (30 degrees), the obtained crude product is added with 1% (wt / wt) of phenothiazine and then subjected to vacuum distillation (oil pump, thorn-type fractionating column, fraction temperature 75-80 degrees, oil temperature 130 degrees) to obtain colorless Liquid 1340g (central control 2)
[0078] (3) The processing method o...
Embodiment 3
[0081] (1) The reaction equation is as follows:
[0082]
[0083] (2) Specific process steps:
[0084] Under the protection of nitrogen, add 712g of tetrahydrofuran to the reaction flask, cool to -25--15 degrees, then add 572g of n-butyl lithium (n-butyl lithium solution with a density of 0.65), and control the internal temperature to not exceed -15 degrees. , Add it in about 15 minutes. Then, after dissolving 216 g of 1-isopropylcyclohexanol in 445 g of tetrahydrofuran, slowly drip into the reaction flask, control the internal temperature not to exceed -20 degrees, and finish the drip in about 40 minutes. After dripping, the reaction flask was heated to 20-25 degrees and continued to stir for 1 hour, and then re-cooled to -5-0 degrees, 346g of methacrylic anhydride was dropped into the reaction flask, control the internal temperature not to exceed 0 degrees, about 40 minutes Finish dripping. Remove the ice bath, raise to room temperature (20-25 degrees) and continue to react fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com