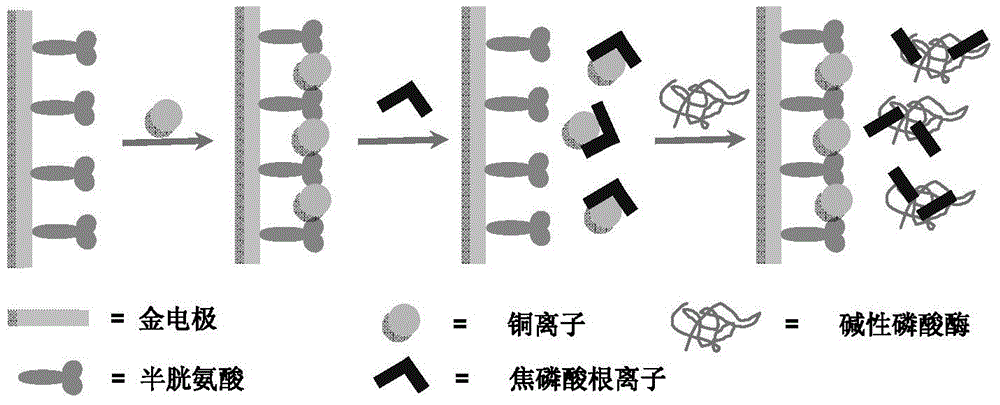
Electrochemical method used for measuring activity of alkaline phosphatase
A phosphatase activity, electrochemical technology, applied in the field of medical testing, to achieve the effect of small sample demand, low cost and accurate measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of the gold electrode modified by embodiment 1, cysteine and copper ions
[0040] 1) Gold electrode pretreatment and activation in sulfuric acid solution
[0041] A gold electrode with a diameter of 1.5mm was used as the working electrode, and the electrode was polished and cleaned before use. The specific process is as follows: the electrode is firstly polished and polished with aluminum oxide powder slurry with a diameter of 0.3 μm and 0.05 μm on the polishing cloth, and then cleaned with secondary deionized water, and ultrasonicated in secondary deionized water and ethanol respectively. Treat for 5 minutes to remove residual Al2O3 powder, and finally clear it with secondary deionized water, and dry it under nitrogen for use. The treated gold electrode was subjected to continuous cyclic voltammetry scanning in 5mL of 0.5M sulfuric acid solution, and the scanning potential window was 0.3V-1.7V.
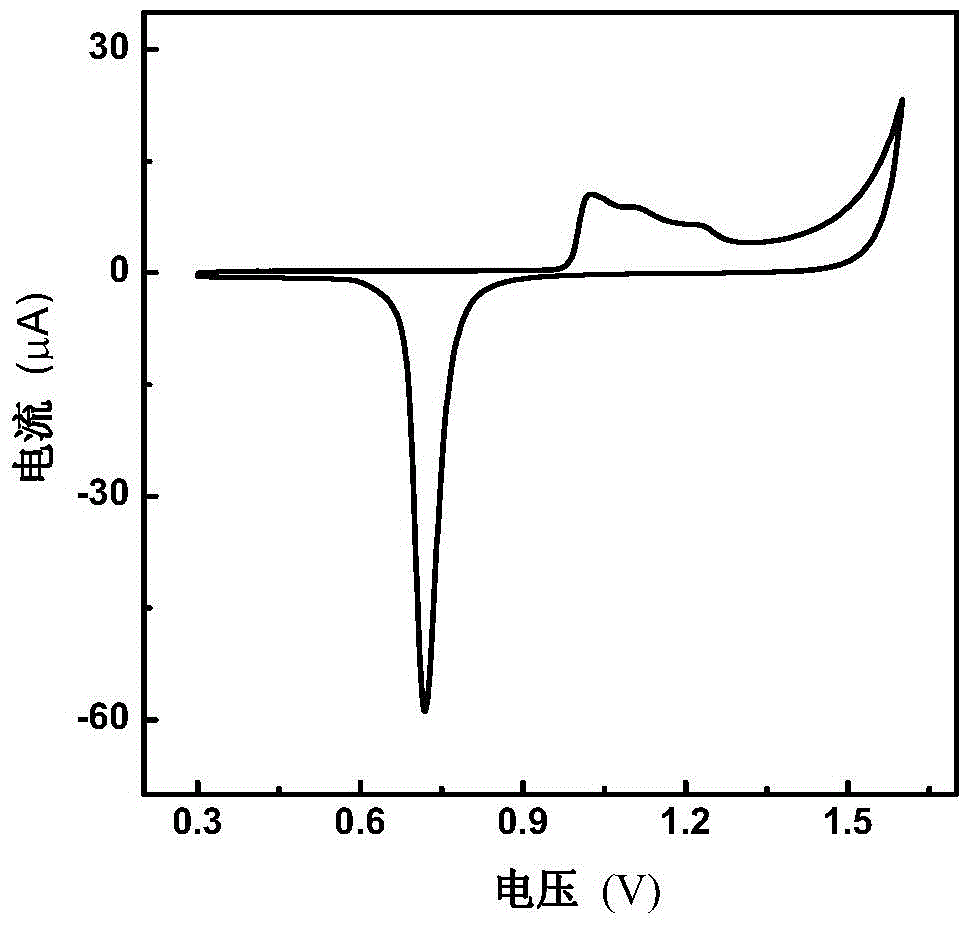
[0042] figure 2 It is the cyclic voltammetry curve of g...
Embodiment 2
[0047] Example 2, Study on the Selectivity of Cysteine and Copper Ion Modified Gold Electrodes to Different Kinds of Anions
[0048] The gold electrode modified by cysteine and copper ions was placed in 4 mL of 0.05 M phosphate buffer solution for cyclic voltammetry scanning, and after stabilization, 1 mL of 20 μM F - , Cl - 、Br - , CO 3 2- 、HCO 3 - , SO 4 2- 、CH 3 COO - , NO 3 - 、H 2 PO 4 - 、HPO 4 2- , PPi and other different kinds of anions were scanned by cyclic voltammetry.
[0049] Figure 5 The selectivity of the gold electrode modified by the cysteine and copper ions to different kinds of anions.
[0050] Depend on Figure 5 It can be seen that the prepared gold electrode modified by cysteine and copper ions has good selectivity to pyrophosphate ion (PPi).
Embodiment 3
[0051] The standard curve of embodiment 3, gold electrode modified by cysteine and copper ions responding to pyrophosphate ions
[0052] The cysteine and copper ion-modified gold electrodes were placed in 4 mL of 0.05 M phosphate buffer solution for cyclic voltammetry scanning, and after stabilization, 1 mL of 1 μM pyrophosphate ion solution and 1 mL of 5 μM pyrophosphate Ion solution, 1 mL of 10 μM pyrophosphate ion solution, 1 mL of 15 μM pyrophosphate ion solution, 1 mL of 20 μM pyrophosphate ion solution, 1 mL of 25 μM pyrophosphate ion solution, and 1 mL of 30 μM pyrophosphate ion solution, stirred Evenly, the detection solution was obtained, left for 5 min, and subjected to cyclic voltammetry scanning. Figure 6 The standard curve for the pyrophosphate ion response of the cysteine and copper ion modified gold electrode.
[0053] Depend on Figure 6 It can be seen that when the concentration of pyrophosphate ions added is in the range of 5 μM to 25 μM (that is, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Molar concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com