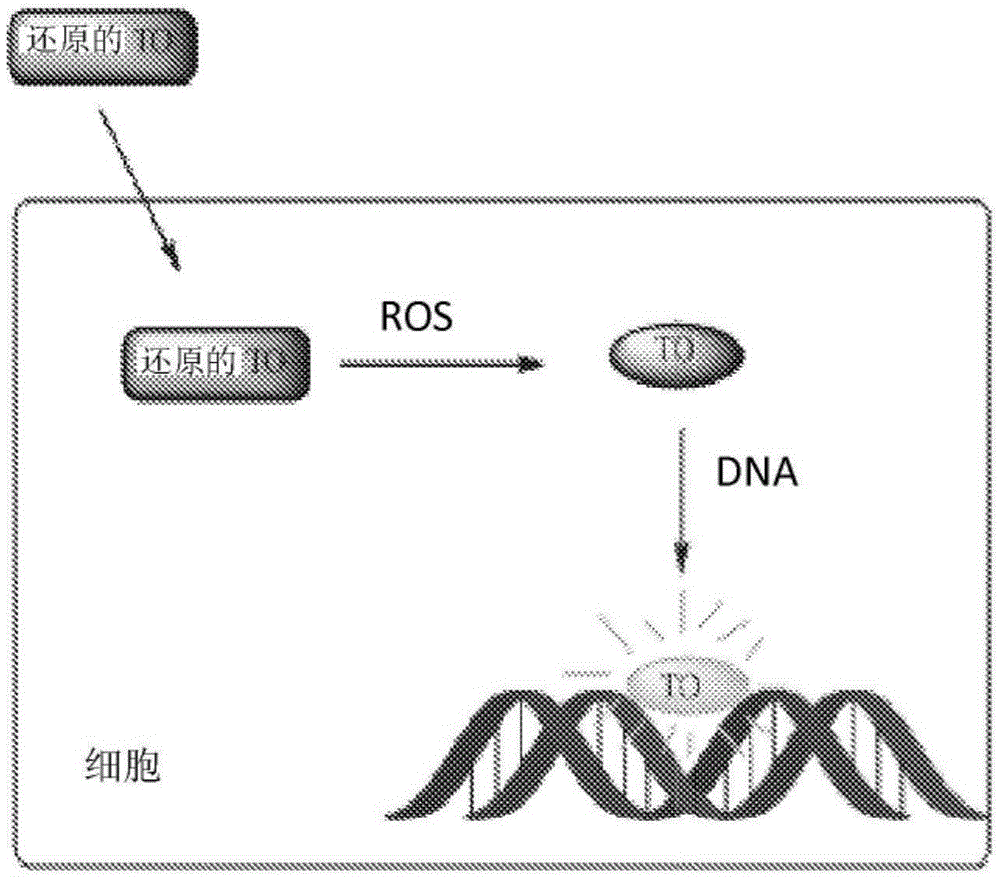
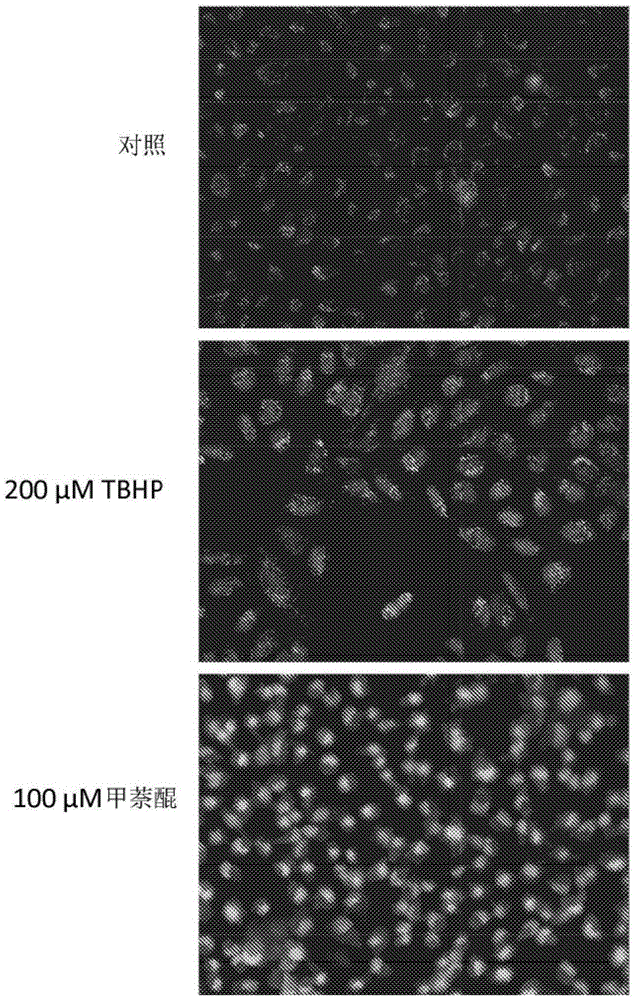
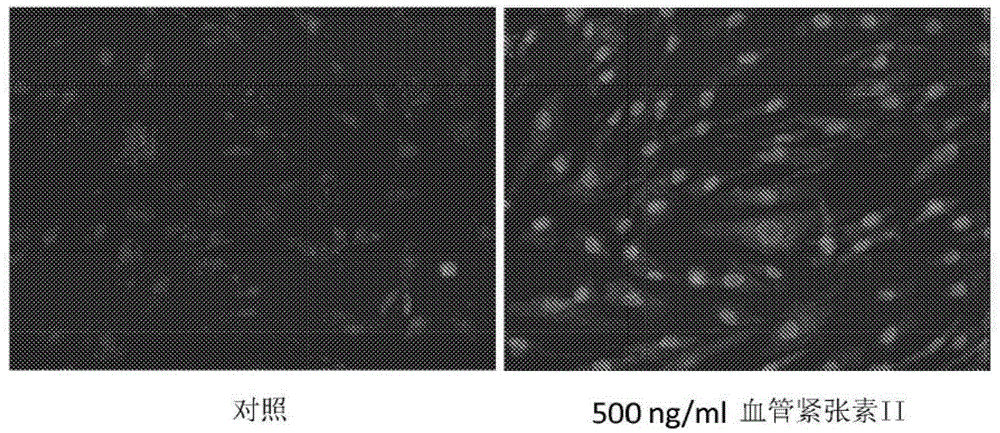
Modified nucleic acid-binding cyanine dyes for detection of reactive oxygen species
A dye compound, structural formula technology, applied in the direction of organic dyes, methine/polymethine dyes, analytical materials, etc., can solve the problems of high toxicity, low emission wavelength, limited application and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Example 1: (Z)-2-((2-butyl-1-phenylquinoline-4(1H)-ylidene)methyl)-3-methyl-2,3-di Synthesis of Hydrobenzo[d]thiazole (1)
[0269]
[0270] To (Z)-2-((2-butyl-1-phenylquinolin-4(1H)-ylidene)methyl)-3-methylbenzo[d]thiazol-3-ium iodide ( To a solution of 350 mg, 0.636 mmol) in methanol (15 mL), sodium borohydride (96 mg, 2.54 mmol) was added slowly, and the mixture was kept stirring at ice-water bath temperature for 30 min. The reaction mixture was diluted with ethyl acetate (150 mL) and washed with water (2x50 mL). The separated organic layer was subjected to Na 2 SO 4 Dry and filter. After evaporation of the solvent, the crude product was purified by column chromatography on silica gel eluting with 2% ethyl acetate in hexanes to afford the desired product ( 1 , 225 mg, 83% yield). TLC: R f =0.45 (silica gel, 5% ethyl acetate in hexane).
Embodiment 2
[0271] Example 2: (Z)-2-((2-butyl-1-phenylquinoline-4(1H)-ylidene)methyl)-3-methyl-2-deuterium-3- Synthesis of Hydrobenzo[d]thiazole (2)
[0272]
[0273] To (Z)-2-((2-butyl-1-phenylquinolin-4(1H)-ylidene)methyl)-3-methylbenzo[d]thiazol-3-ium iodide ( To a solution of 100 mg, 0.18 mmol) in methanol (5 mL), sodium borodeuteride (22 mg, 0.53 mmol) was added slowly, and the mixture was kept stirring at ice-water bath temperature for 30 min. The reaction mixture was diluted with ethyl acetate (50 mL) and washed with water (2x30 mL). The separated organic layer was subjected to Na 2 SO 4 Dry and filter. After evaporation of the solvent, the crude product was purified by column chromatography on silica gel eluting with 2% ethyl acetate in hexanes to afford the desired product ( 2 , 50 mg, 65% yield). TLC: R f =0.43 (silica gel, 5% ethyl acetate in hexane).
Embodiment 3
[0274] Example 3: (Z)-3-methyl-2-((1-propylquinoline-4(1H)-ylidene)methyl)-2,3-dihydrobenzo[d] Synthesis of Thiazole (3)
[0275]
[0276] with the above compound 1 A similar procedure described in the Examples, from (Z)-3-methyl-2-((1-propylquinolin-4(1H)-ylidene)methyl)benzo[d]thiazole-3 -onium tosylate and sodium borohydride preparation compound 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com