Synthesis process of 4, 4'-dichlorodiphenyl sulfone
A technique for the synthesis of dichlorodiphenyl sulfone, which is applied in the field of synthesis of 4,4'-dichlorodiphenyl sulfone, can solve the problems of low cost of sulfuric acid process, low melting point of products, high production cost, and easy post-processing , low cost, less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
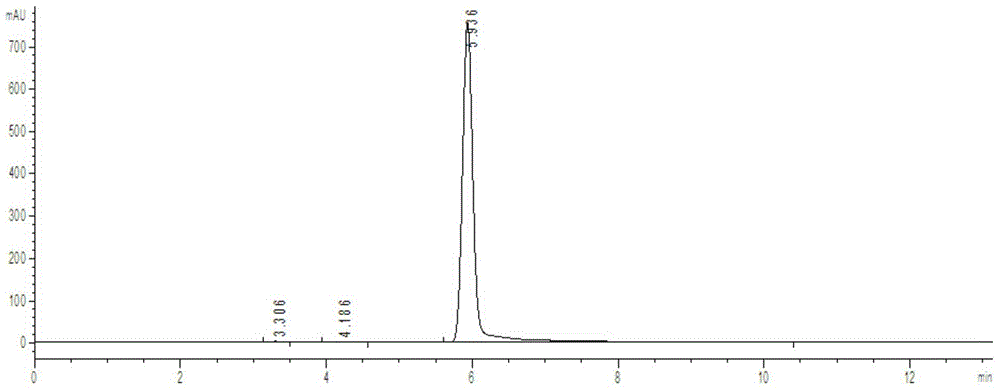
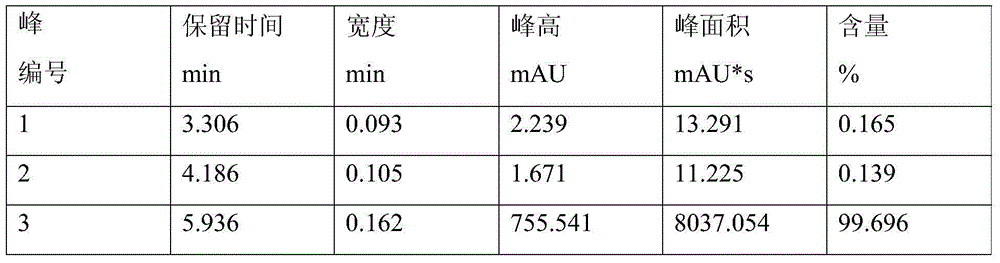
[0022] First add 112.5 g of chlorobenzene into the four-neck flask, then add 26.7 g of anhydrous aluminum trichloride, stir well, then add 23.8 g of thionyl chloride dropwise, the reaction is exothermic, and the temperature is controlled at 30°C. After adding the thionyl chloride dropwise, keep stirring for 1 hour. After the reaction, the temperature is lowered to 15°C for hydrolysis, and the heat is released by hydrolysis. The temperature is controlled at 45°C. After 0.5h, the hydrolysis was completed, the temperature was raised to 100°C, and the temperature was kept at reflux for 0.5h, then the temperature was lowered and cooled to 15°C, and the solid was filtered to obtain 51.2g of 4,4'-dichlorodiphenylsulfoxide. Heat to dissolve with glacial acetic acid, add hydrogen peroxide dropwise to oxidize at 60°C, keep warm and oxidize for 2 hours, cool down and filter to obtain 51.9g of 4,4'-dichlorodiphenylsulfone, the chromatographic content is 99.2%, and the yield is 90.4%.
Embodiment 2
[0024] First add 150 g of chlorobenzene into the four-neck flask, then add 26.7 g of anhydrous aluminum trichloride, stir well, then add 25 g of thionyl chloride dropwise, the reaction is exothermic, and the temperature is controlled at 27°C. After adding the thionyl chloride dropwise, keep stirring for 2 hours. After the reaction, the temperature is lowered to 15°C for hydrolysis, and the heat is released from the hydrolysis. The temperature is controlled at 30°C. After 1 hour, the hydrolysis was completed, the temperature was raised to 110°C, and the temperature was kept at reflux for 1 hour, then the temperature was lowered and cooled to 20°C, and 52.3 g of 4,4'-dichlorodiphenylsulfoxide was obtained by filtration. Heat to dissolve with glacial acetic acid, add hydrogen peroxide dropwise to oxidize at 80°C, keep warm and oxidize for 1 hour, cool down and filter to obtain 52.4g of 4,4'-dichlorodiphenylsulfone, the chromatographic content is 99.4%, and the yield is 91.0%.
Embodiment 3
[0026] First add 198g of chlorobenzene into the four-neck flask, then add 26.7g of anhydrous aluminum trichloride, stir well, then add 26.2g of thionyl chloride dropwise, the reaction exotherms, and the temperature is controlled at 23°C. After adding the thionyl chloride dropwise, keep stirring for 1.5 hours. After the reaction, the temperature is lowered to 15°C for hydrolysis, and the heat is released from the hydrolysis. The temperature is controlled at 20°C. After 1.5 hours, the hydrolysis was completed, the temperature was raised to 120°C, and the temperature was kept at reflux for 1 hour, then the temperature was lowered and cooled to 25°C, and 51.9 g of 4,4'-dichlorodiphenylsulfoxide was obtained by filtration. Heat to dissolve with glacial acetic acid, add hydrogen peroxide dropwise to oxidize at 70°C, keep warm and oxidize for 1 hour, cool down and filter to obtain 51.8g of 4,4'-dichlorodiphenylsulfone, the chromatographic content is 99.7%, and the yield is 90.2%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com