Pyrrole ring containing hemifumarate as proton pump inhibitor as well as intermediate and pharmaceutical application thereof
A technology of hemi-fumarate and fumaric acid, applied in the field of medicine and biology, can solve problems such as ulcers, and achieve the effects of good stability, good gastric acid inhibition and excellent biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
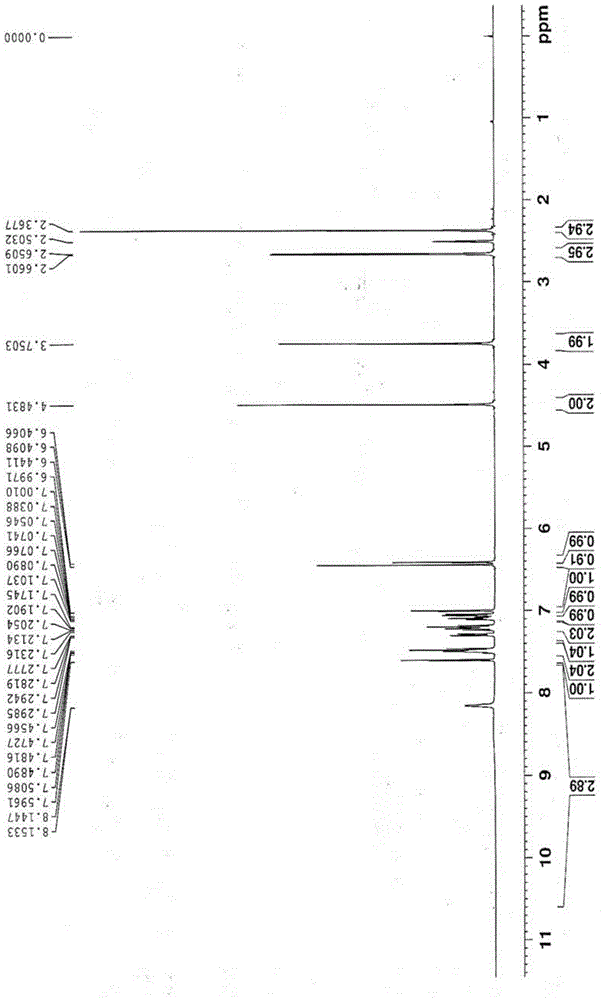
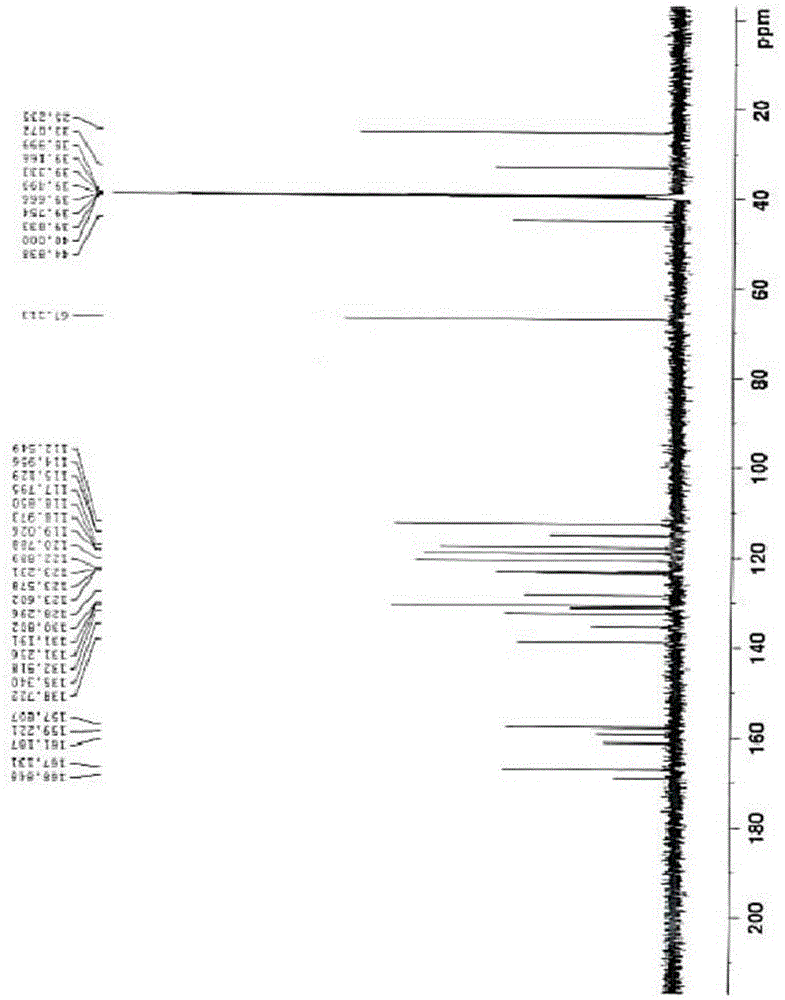
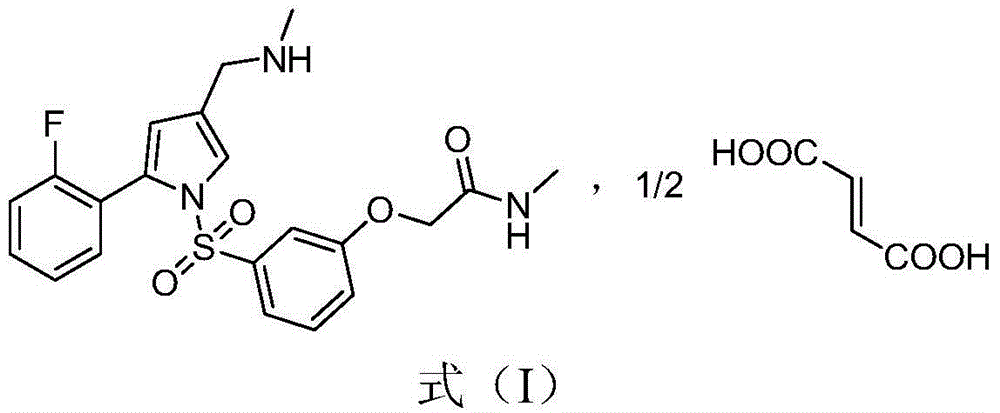
[0033] 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N-methyl Acetamide 10g was dissolved in a mixed solvent of isopropanol (80ml) and absolute ethanol (20ml), and 1.26g of fumaric acid was added with stirring, then heated to 50°C and refluxed for 30 minutes. Naturally cooled to room temperature, stirred for 2 hours, filtered, and dried to obtain 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl) Sulfonyl)phenoxy)-N-methylacetamide hemifumarate.
Embodiment 2
[0035] 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N-methyl Acetamide 10g, dissolved in isopropanol (80ml), added fumaric acid ethanol solution (1.26g / 20ml), heated to reflux, all solids dissolved, naturally cooled to room temperature under stirring conditions, stirred for 2 hours, filtered, Drying gave 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N-methyl Acetamide hemifumarate.
Embodiment 3
[0036] Example 3: 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N -Preparation of methylacetamide hemifumarate intermediate and free base
[0037]
[0038] first step
[0039] tert-Butyl((5-(2-fluorophenyl)-1H-pyrrol-3-yl)methyl)(methyl)carbonate (3.0g, 10mmol) and 3-(2-(methylamino)- Add 2-oxoethoxy)phenyl-1-sulfonyl chloride (2.64mg, 10mmol) into acetonitrile, add DIEA and stir for 2-5 hours. The temperature of the reaction solution was cooled, dilute hydrochloric acid was added to adjust the pH to 4-5, and purified water was added for crystallization to obtain compound III, 4.9 g, yield: 92.3%.
[0040] second step
[0041]Compound III (4.5 g) was dissolved in 15 mL of ethyl acetate, the temperature of the reaction liquid was cooled to 10° C. in an ice bath, hydrochloric acid gas was added, and the reaction was stirred for 1 hour. Use sodium bicarbonate to adjust pH to alkaline, wash with saturated sodium chloride solution, dry ov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com