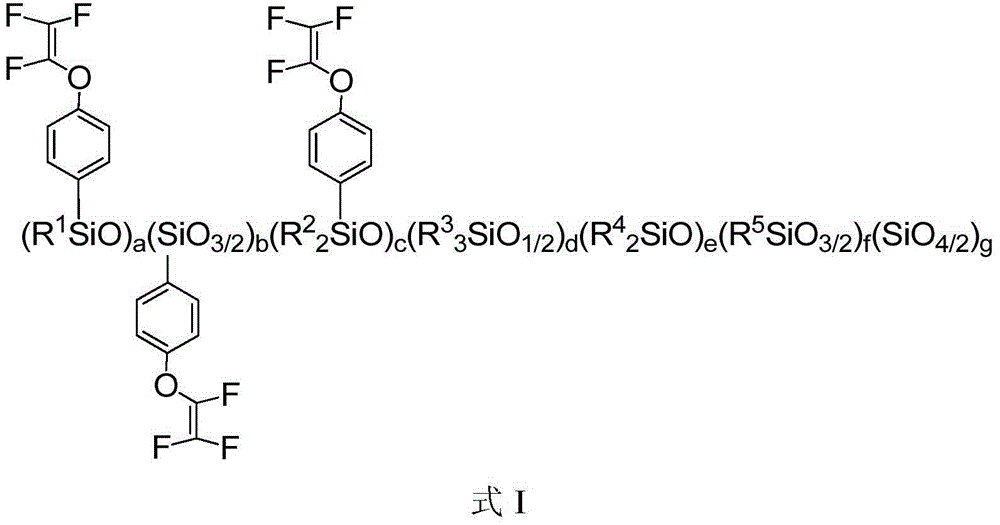Preparation method and application of trifluoroethyleneoxyphenyl-containing silicone resin
A technology of trifluorovinyloxyphenyl and dimethoxysilane, which is applied in the field of high-performance polymer manufacturing and can solve problems such as decreased adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0108] Described organosilicon resin can be prepared by following preparation method:
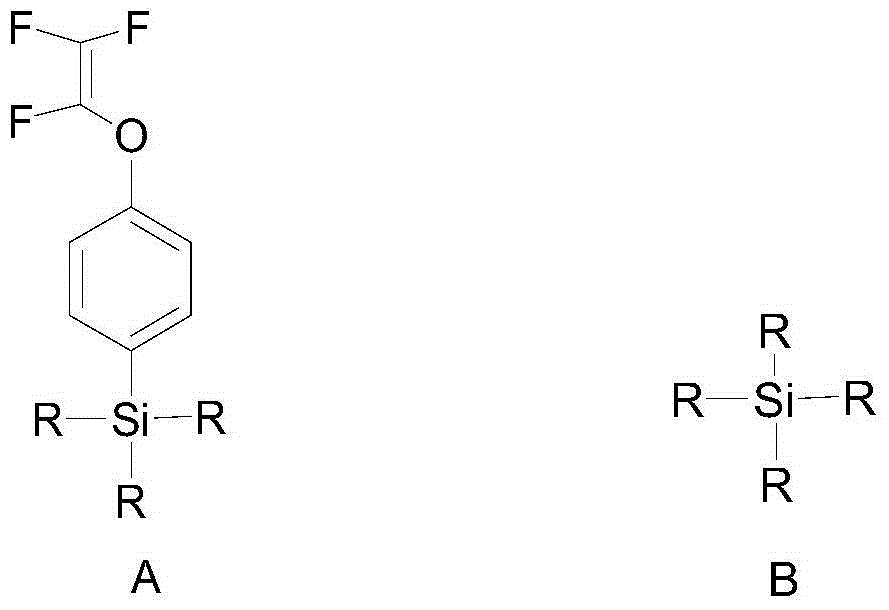
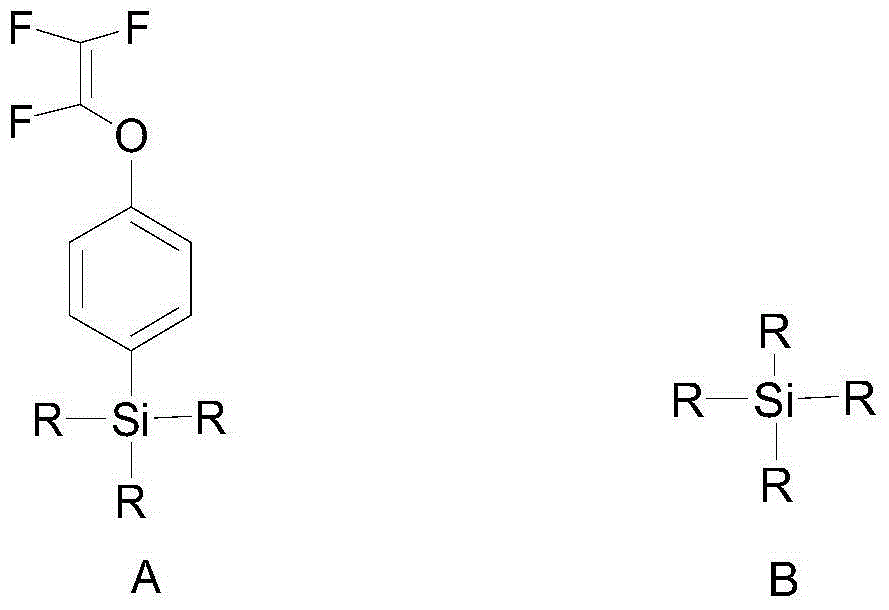
[0109] In an inert solvent, carry out hydrolytic polymerization with an alkoxysilane monomer containing trifluorovinyloxyphenyl selected from the group (a) and an alkoxysilane monomer selected from the group (b) to obtain the following: The organosilicon resin shown in formula I; Described group (a) comprises: methyl (trifluorovinyloxyphenyl) dimethoxysilane, trifluorovinyloxyphenyl trimethoxysilane, methyl ( Trifluorovinyloxyphenyl)diethoxysilane, trifluorovinyloxyphenyltriethoxysilane, or combinations thereof; the group (b) includes: ethyl orthosilicate, phenyltrimethoxy phenylsilane, methylphenyldimethoxysilane, phenyltriethoxysilane, diphenyldimethoxysilane, trimethylethoxysilane, dodecyltrimethoxysilane, or combinations thereof .
[0110] The hydrolysis polymerization reaction is preferably carried out in the presence of an acidic catalyst and / or water; preferably, the acidic catalys...
Embodiment 1
[0135] Example 1 Preparation of organosilicon resin I containing trifluoroethyleneoxyphenyl
[0136] Add 50 milliliters of water, 50 milliliters of concentrated hydrochloric acid and 30 milliliters of toluene into a clean 500 milliliter three-necked bottle, and add 3.6 grams of tetraethyl orthosilicate, 30.0 grams of phenyltrimethoxysilane, A mixture of 17.0 grams of methylphenyldimethoxysilane, 9.8 grams of methyl (trifluorovinyloxyphenyl) dimethoxysilane, 10.2 grams of trifluorovinyloxyphenyltrimethoxysilane and 20 milliliters of toluene Solution, the reaction temperature is controlled below 20°C, continue to react for 3h after the dropwise addition, then heat to 80°C for 3h, cool to room temperature, wash with water until neutral, dry the organic layer with anhydrous sodium sulfate, remove toluene after filtration , under reduced pressure and heated to 150° C. / 10 mba to remove small molecule compounds, and obtain 17.1 g of trifluoroethyleneoxyphenyl-containing organosilicon...
Embodiment 2
[0137] Example 2 Preparation of organosilicon resin II containing trifluoroethyleneoxyphenyl
[0138] Add 20 milliliters of water, 50 milliliters of acetic acid and 30 milliliters of toluene into a clean 500 milliliter three-necked bottle, and add 3.8 grams of orthosilicate, 41.3 grams of phenyltriethoxysilane, A mixture of 18.5 grams of methylphenyldimethoxysilane, 9.2 grams of methyl (trifluorovinyloxyphenyl) dimethoxysilane, 10.5 grams of trifluorovinyloxyphenyltrimethoxysilane and 20 milliliters of toluene Solution, the reaction temperature is controlled below 20°C, after the dropwise addition, heat to 100°C to continue the reaction for 16 h, cool to room temperature, wash with water until neutral, dry the organic layer with anhydrous sodium sulfate, remove toluene after filtration, and raise the temperature under reduced pressure Remove the small molecular compound at 150°C / 10mba to obtain 19.3 g of organosilicon resin II containing trifluoroethyleneoxyphenyl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com