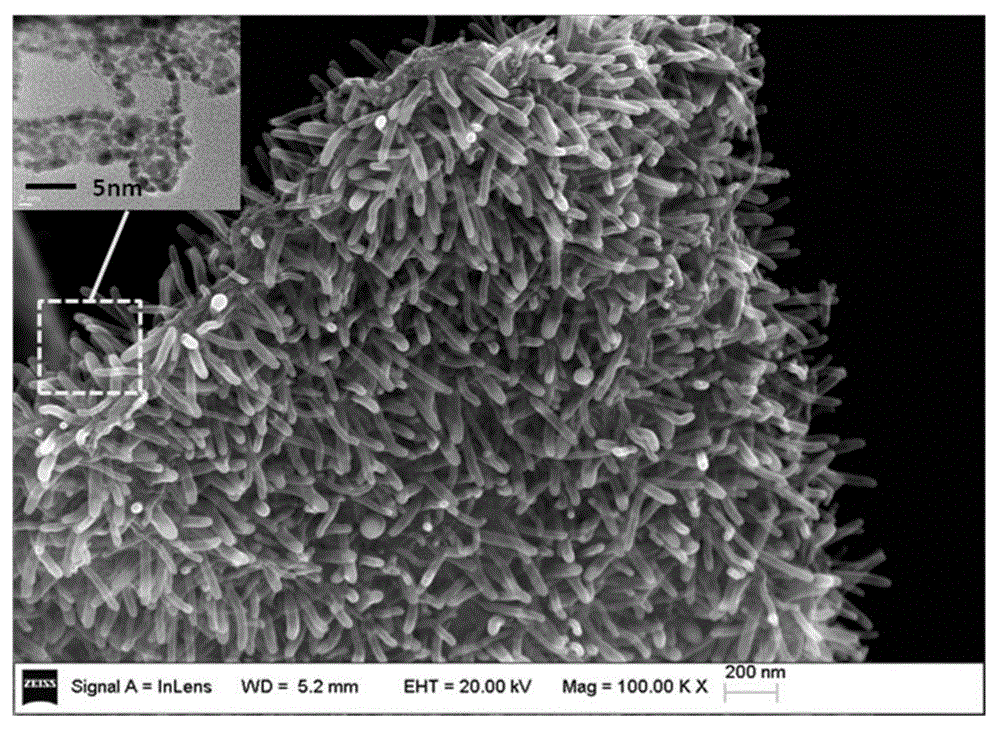
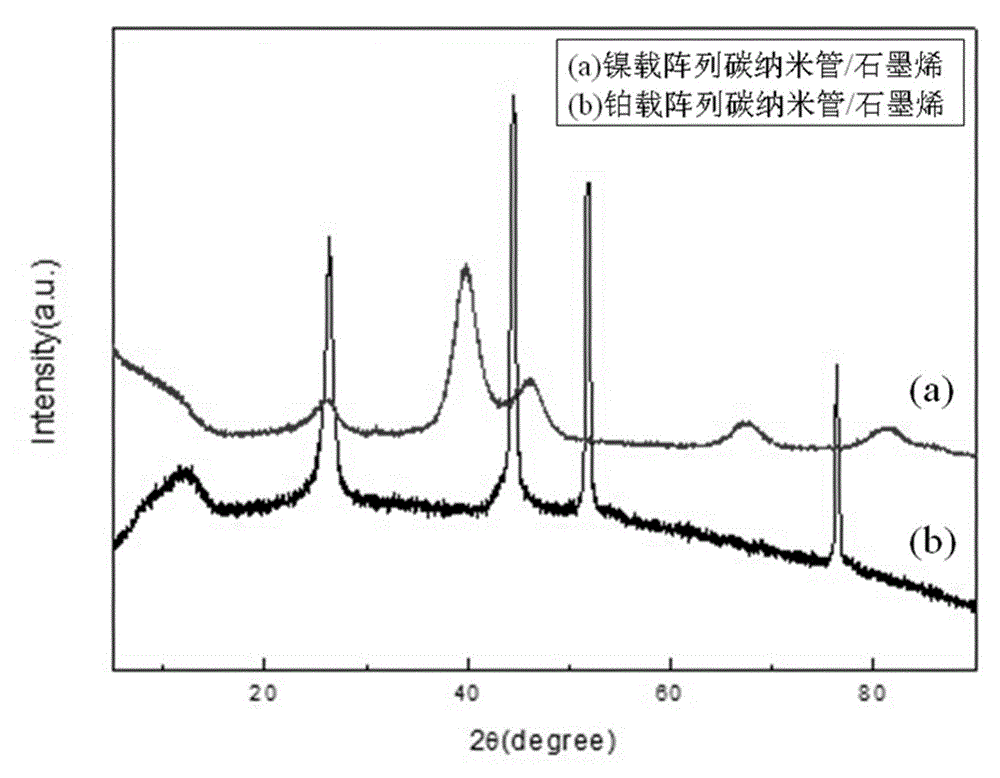
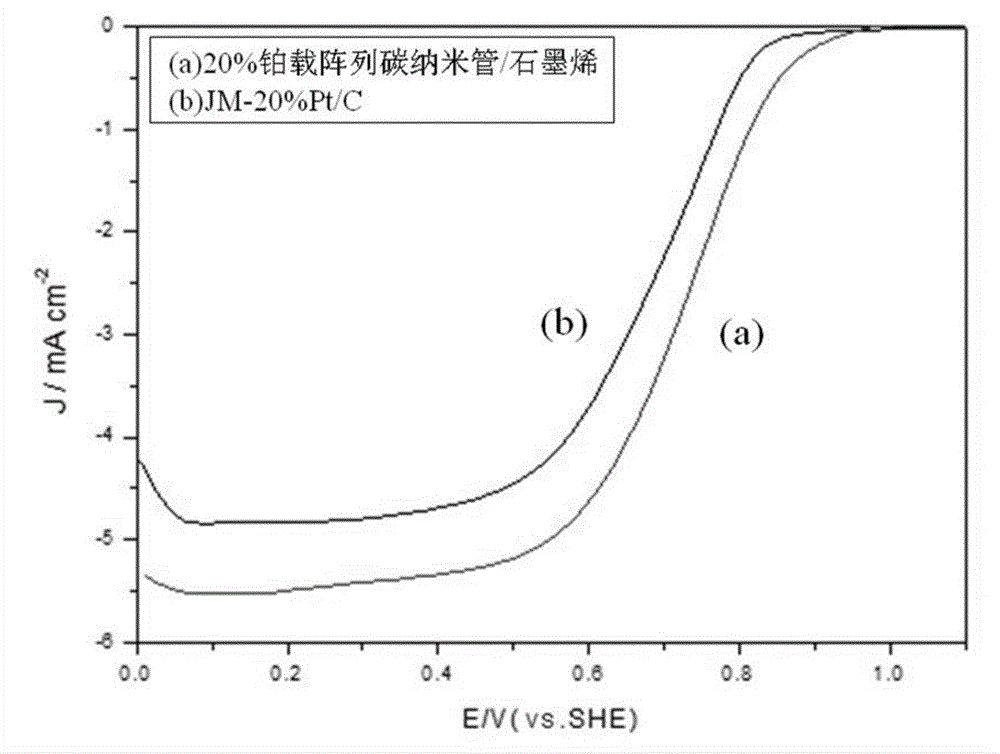
Array carbon nano-tube/graphene platinum-supported catalyst for fuel cell and preparation method of array carbon nano-tube/graphene platinum-supported catalyst
A carbon nanotube and fuel cell technology, applied in the field of electrochemistry, can solve problems such as shedding, large catalyst particle size, and reduced catalytic efficiency, and achieve the effects of strong electrical conductivity, large specific surface area, and increased mass transfer rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Take 3g of flake graphite, add concentrated sulfuric acid and concentrated phosphoric acid respectively, and pre-oxidize for 24h; slowly add an appropriate amount of potassium permanganate 18g under ice bath conditions, and control the temperature below 20°C; then heat and react at 50°C for 12h, and wait for The reactant was cooled to room temperature, poured into 400ml of ice-deionized water, added 10ml of 30% hydrogen peroxide, first centrifugally washed with 5% HCl, and then centrifugally washed with deionized water to obtain wet graphite oxide;
[0031] (2) Take 100ml of wet graphite oxide obtained in step 1, put it into 500ml of deionized water, and continue ultrasonication for 3 hours to fully dissolve it. After ultrasonication, add 10ml of hydrazine hydrate, reflux at 90°C for 6h, cool, filter and wash the obtained product, and freeze-dry it. The obtained product is graphene (RGO);
[0032] (3) get the Graphene 0.1g that obtains in step 2 and carry out sensit...
Embodiment 2
[0036](1) Take 3g of flake graphite, add 360ml and 40ml of concentrated sulfuric acid and concentrated phosphoric acid respectively, and pre-oxidize for 24 hours; slowly add an appropriate amount of potassium permanganate 18g under ice bath conditions, and control the temperature below 20°C; then heat the reaction at 50°C After 12 hours, the reactants were cooled to room temperature, poured into 400ml ice deionized water, added 10ml 30% hydrogen peroxide, first centrifugally washed with 5% HCl, and then centrifugally washed with deionized water to obtain wet graphite oxide;
[0037] (2) Take 100ml of wet graphite oxide obtained in step 1, put it into 500ml of deionized water, and continue ultrasonication for 3 hours to fully dissolve it. After ultrasonication, add 10ml of hydrazine hydrate, reflux at 90°C for 6h, cool, filter and wash the obtained product, and freeze-dry it. The obtained product is graphene (RGO);
[0038] (3) get the Graphene 0.1g that obtains in step 2 and c...
Embodiment 3
[0042] (1) Take 3g of flake graphite, add 360ml and 40ml of concentrated sulfuric acid and concentrated phosphoric acid respectively, and pre-oxidize for 24 hours; slowly add an appropriate amount of potassium permanganate 18g under ice bath conditions, and control the temperature below 20°C; then heat the reaction at 50°C After 12 hours, the reactants were cooled to room temperature, poured into 400ml ice deionized water, added 10ml 30% hydrogen peroxide, first centrifugally washed with 5% HCl, and then centrifugally washed with deionized water to obtain wet graphite oxide;
[0043] (2) Take 100ml of wet graphite oxide obtained in step 1, put it into 500ml of deionized water, and continue ultrasonication for 3 hours to fully dissolve it. After ultrasonication, add 10ml of hydrazine hydrate, reflux at 90°C for 6h, cool, filter and wash the obtained product, and freeze-dry it. The obtained product is graphene (RGO);
[0044] (3) get the Graphene 0.1g that obtains in step 2 and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com