A kind of three-dimensional carbon nanomaterial and its preparation method and application
A carbon nanomaterial, three-dimensional technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the high requirements of water treatment process, troublesome separation and desorption treatment, difficult to process modular products, etc. It is easy to achieve large-scale production, promote industrialization and large-scale development, and the preparation process is simple and controllable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
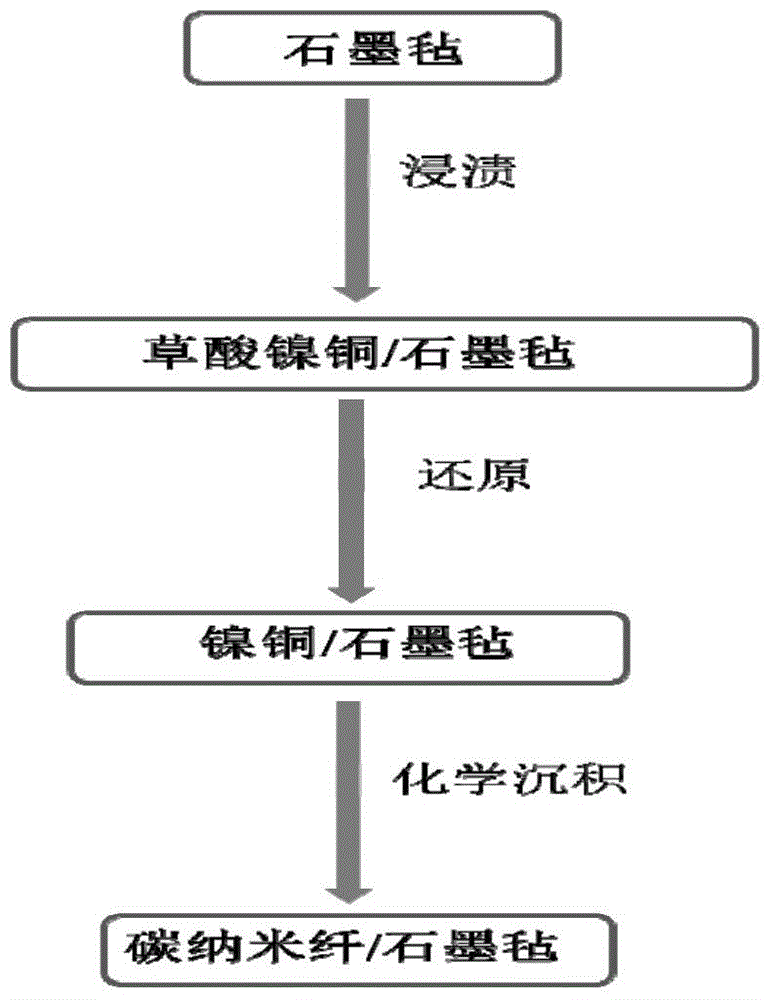
[0035] (1) Preparation of catalyst precursor (nickel-copper oxalate nanofibers):
[0036] Take 2.6g of nickel nitrate hexahydrate and 0.7g of nickel-copper trihydrate respectively, dissolve them in 30mL of ethanol to form solutions A and B, then titrate solution B in solution A under magnetic stirring, and transfer the obtained suspension to Store in an autoclave at 120°C for 12 hours. The finally obtained product was rinsed with deionized water and dried at 80° C. to obtain nickel copper oxalate.
[0037] (2) Transfer of the catalyst precursor to the graphite felt substrate: the graphite felt was first pretreated by thermal activation at 420°C for 12 hours in air; nickel-copper oxalate was deposited on the graphite felt substrate by the impregnation method. Disperse 0.2 g of nickel-copper oxalate into 60 mL of ethanol by alternating sonication to form a dispersion suspension. The activated graphite felt was immersed in the obtained suspension for 15 min, and then the graphi...
Embodiment 2
[0041] Preparation of catalyst precursor (iron oxide nanorods):
[0042] (1) Take 3.3g of ferric nitrate and 1.2g of sodium hydroxide respectively, dissolve them in 30mL of ethanol to form solutions A and B, then titrate solution B in solution A under magnetic stirring, and transfer the obtained suspension to high pressure Kettle, 120 ℃ for 12h. The finally obtained product was rinsed with deionized water, dried at 80° C., and calcined in air for 4 hours to obtain iron oxide nanorods.
[0043](2) Transfer of the catalyst precursor to the graphite felt substrate: the graphite felt was first pretreated by thermal activation at 420°C for 12 hours in air; the iron oxide nanorods were deposited on the graphite felt substrate by impregnation. Disperse 0.2 g of iron oxide nanorods into 60 mL of ethanol by alternating sonication to form a dispersion suspension. The activated graphite felt was immersed in the obtained suspension for 15 min, and then the graphite felt was taken out an...
Embodiment 3
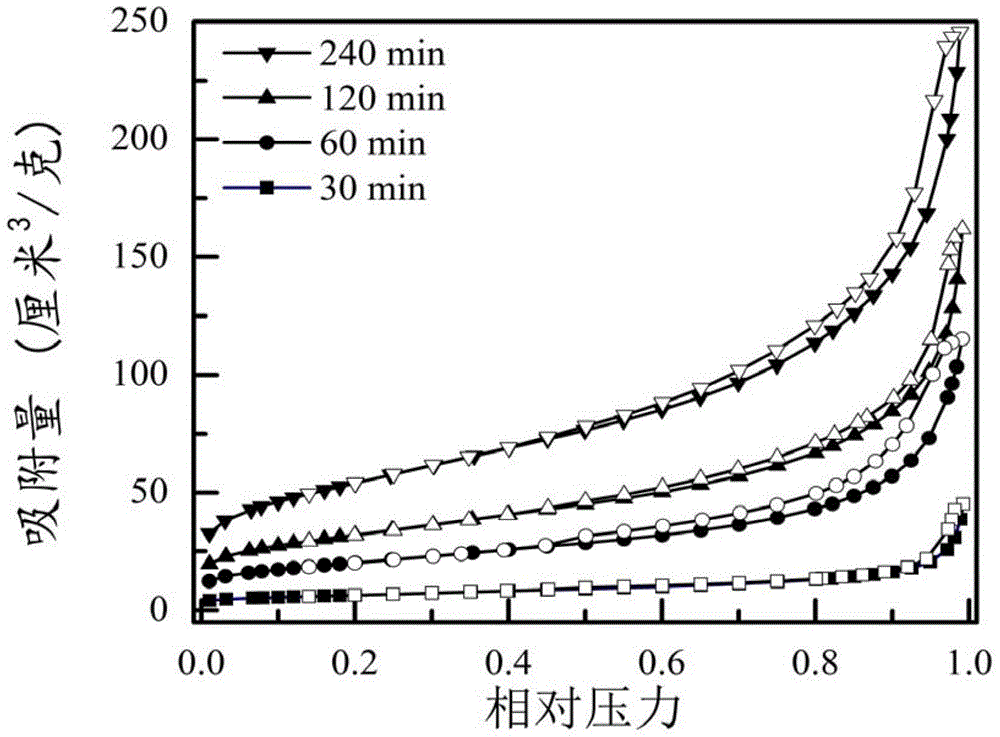
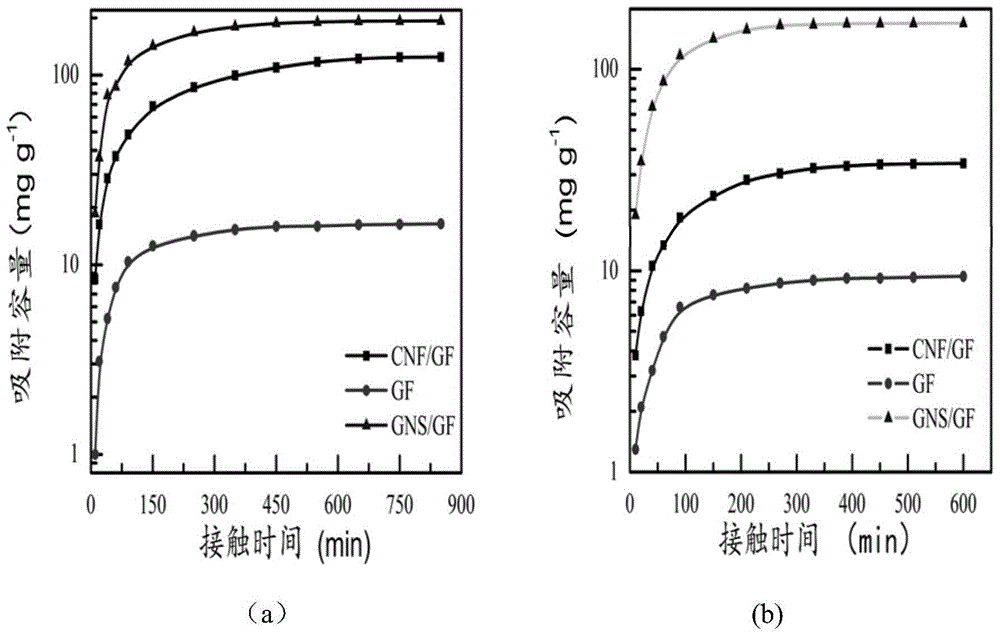
[0047] For the preparation of CNF / GF compound, control its reaction time to be 60min, the preparation method is the same as embodiment 1, and the compound GNF / GF that the reaction time is 60min is obtained, and the BET surface area of embodiment 3 is 72.2m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com