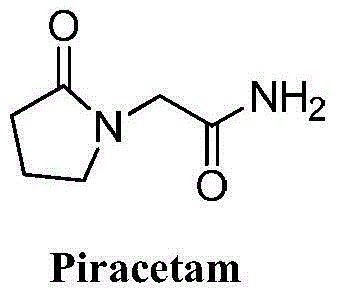
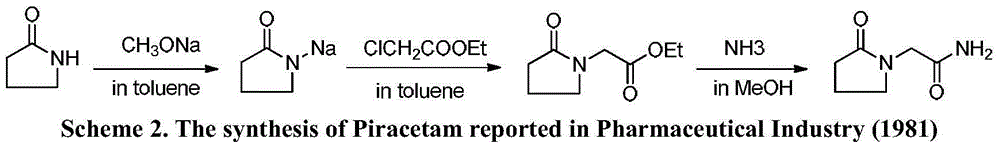
Novel synthesis method of nootropic piracetam
A technology of piracetam and a synthesis method, applied in the field of piracetam synthesis, can solve the problems of affecting the reaction speed, easy to absorb moisture and caking, difficult to realize industrialized production and the like, and achieve mild reaction conditions, simple and convenient production operation, and environmental protection. friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The method for synthesizing piracetam, it may further comprise the steps:
[0037] Preparation of α-pyrrolidone sodium salt: Equip a 1000mL three-necked bottle with mechanical stirring, a constant pressure dropping funnel and a thorn-shaped fractionating column. The upper end of the fractionation column is connected with a thermometer, a condenser and a 500mL receiving bottle. Under mechanical stirring, 46 mL (0.60 mol) of α-pyrrolidone and 250 mL of toluene were sequentially added into the three-necked flask. When the temperature of the reaction system reached 70° C., a methanol solution of sodium methoxide (28.4% (w / w)); 114.0 g; 0.60 mol) was added dropwise under reduced pressure, and the distillate was collected. After the dropwise addition was completed, the temperature was raised, and the distillation was carried out at atmospheric pressure until the distillate was completely distilled off, and the reaction was completed.
[0038] Preparation of α-pyrrolidone me...
Embodiment 2
[0042] Preparation of α-pyrrolidone sodium salt: Equip a 1000mL three-necked bottle with mechanical stirring, a constant pressure dropping funnel and a thorn-shaped fractionating column. The upper end of the fractionation column is connected with a thermometer, a condenser and a 500mL receiving bottle. Under mechanical stirring, 46 mL (0.60 mol) of α-pyrrolidone and 250 mL of toluene were sequentially added into the three-necked flask. When the temperature of the reaction system reached 100° C., a methanol solution of sodium methoxide (28.4% (w / w)); 114.0 g; 0.60 mol) was added dropwise under reduced pressure, and the distillate was collected. After the dropwise addition, toluene was added, the temperature was raised, and distillation was carried out at atmospheric pressure until the distillate was completely distilled off, and the reaction was completed.
[0043] Preparation of α-pyrrolidone methyl acetate: remove the fractionation device, connect a thermometer and a condens...
Embodiment 3
[0047] Preparation of α-pyrrolidone sodium salt: Equip a 1000mL three-necked bottle with mechanical stirring, a constant pressure dropping funnel and a thorn-shaped fractionating column. The upper end of the fractionation column is connected with a thermometer, a condenser and a 1000mL receiving bottle. Under mechanical stirring, 46 mL (0.60 mol) of α-pyrrolidone and 250 mL of toluene were sequentially added into the three-necked flask. When the temperature of the reaction system reached 70° C., a methanol solution of sodium methoxide (28.4% (w / w)); 114.0 g; 0.60 mol) was added dropwise under reduced pressure, and the distillate was collected. After the dropwise addition was completed, the temperature was raised, and the distillation was carried out at atmospheric pressure until the distillate was completely distilled off, and the reaction was completed.
[0048] Preparation of α-pyrrolidone methyl acetate: remove the fractionation device, connect a thermometer and a condense...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com