Crystallized mesoporous magnesium silicate powder and preparation method thereof
A technology of crystallizing mesoporous and magnesium silicate, which is applied in the direction of magnesium silicate and silicate, can solve the problems of difficult control of hydrolysis-condensation products, hard template channels, difficult recycling and reuse, etc., and reach the experimental calcination temperature Low, high crystallinity, large pore size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
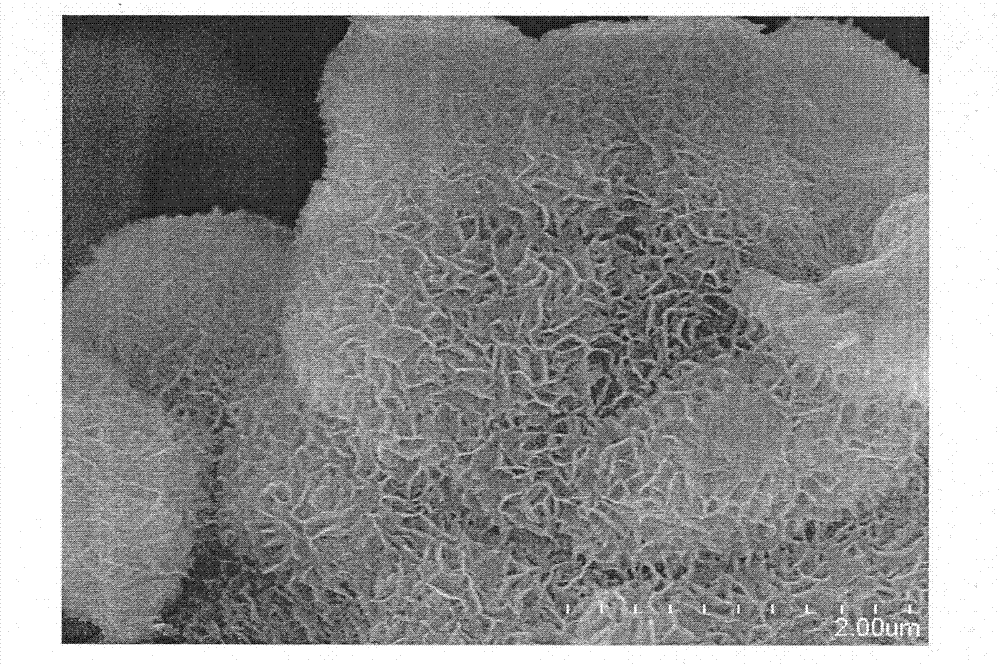
[0020] Embodiment 1, with 0.375mmol MgCl 2 ·6H 2 O with 5mmol NH 4 Cl was dissolved in 50 mL of deionized water, and then 0.5 mL of 28% NH 3 ·H 2 O stir well to make solution a. 0.0387g C@SBA-15 (containing 0.024g SiO 2 ) was dispersed in 25mL deionized water to prepare suspension b. The solution a and the suspension b were mixed and fully stirred, then transferred to a 100mL reaction kettle, and hydrothermally reacted at 140°C for 12h. The product after the hydrothermal reaction was cooled to room temperature, centrifuged and the supernatant was discarded. Wash with deionized water until pH=7. After drying at 100° C. for 4 hours, transfer to a muffle furnace, and calcining at 600° C. for 5 hours to remove C, and obtain crystallized mesoporous magnesium silicate powder. figure 1 It is a scanning electron micrograph of the prepared crystalline mesoporous magnesium silicate powder.
Embodiment 2
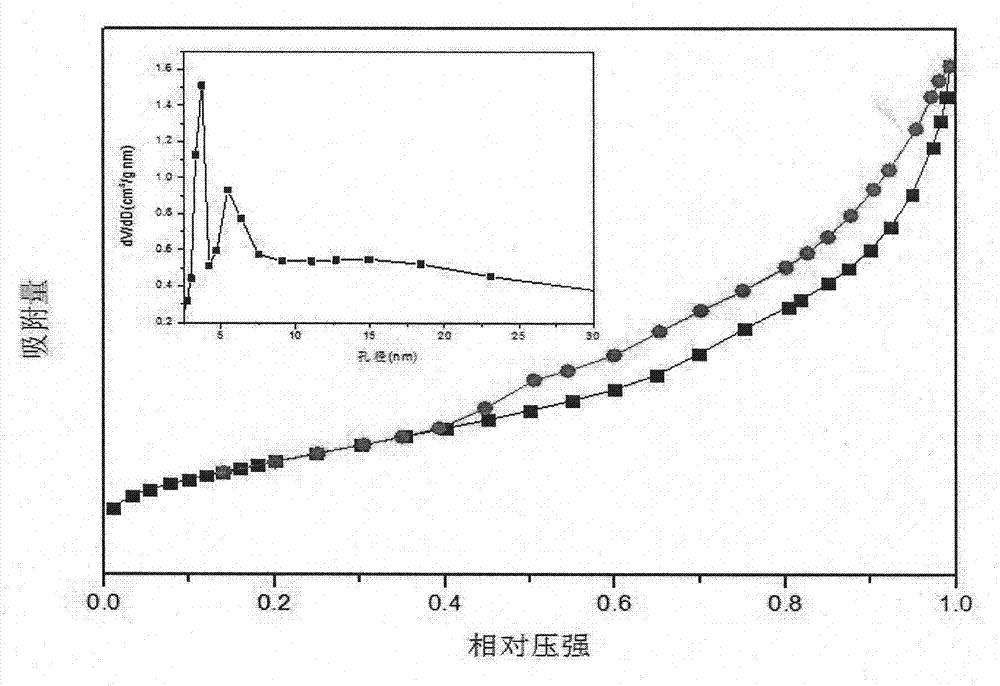
[0021] Embodiment 2, with 0.75mmol MgCl 2 ·6H 2 O with 10mmol NH 4 Cl was dissolved in 50 mL of deionized water, and then 1 mL of 28% NH 3 ·H 2 O stir well to make solution a. 0.0774g C@SBA-15 (containing 0.048g SiO 2 ) was dispersed in 25mL deionized water to prepare suspension b. The solution a and the suspension b were mixed and fully stirred, then transferred to a 100mL reaction kettle, and hydrothermally reacted at 140°C for 12h. The product after the hydrothermal reaction was cooled to room temperature, centrifuged and the supernatant was discarded. Wash with deionized water until pH=7. After drying at 100° C. for 4 hours, transfer to a muffle furnace, and calcining at 600° C. for 5 hours to remove C, and obtain crystallized mesoporous magnesium silicate powder. figure 2 It is the nitrogen absorption and desorption spectrum of the prepared crystalline mesoporous magnesium silicate powder.
Embodiment 3
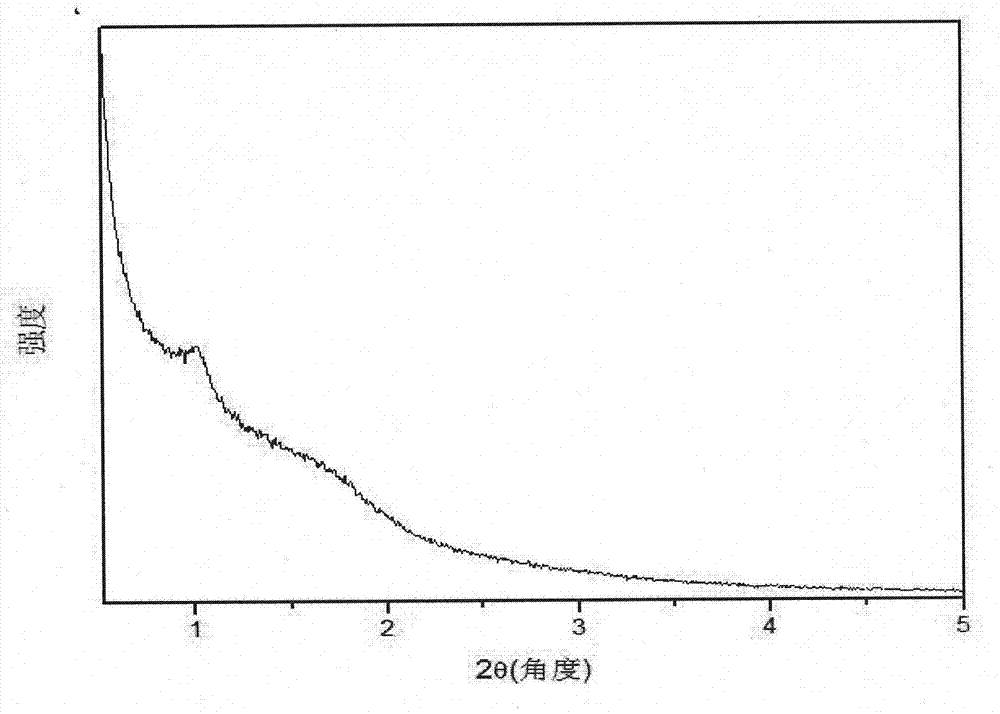
[0022] Embodiment 3, with 1.5mmol MgCl 2 ·6H 2 O with 20mmol NH 4Dissolve Cl in 50 mL of deionized water, then add 2 mL of 28% NH 3 ·H 2 O stir well to make solution a. 0.1548g C@SBA-15 (containing 0.096g SiO 2 ) was dispersed in 25mL deionized water to prepare suspension b. The solution a and the suspension b were mixed and fully stirred, then transferred to a 100mL reaction kettle, and hydrothermally reacted at 140°C for 12h. The product after the hydrothermal reaction was cooled to room temperature, centrifuged and the supernatant was discarded. Wash with deionized water until pH=7. After drying at 100° C. for 4 hours, transfer to a muffle furnace, and calcining at 600° C. for 5 hours to remove C, and obtain crystallized mesoporous magnesium silicate powder. image 3 It is the small-angle X-ray diffraction pattern of the prepared crystalline mesoporous magnesium silicate powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com