Copper coordination polymer having selective ion exchange function and application of copper coordination polymer
A technology of coordination polymers and copper complexes, applied in the field of copper coordination polymers and preparation, Cu-anthracycline bistriazole two-dimensional copper coordination polymers, can solve the problems of rare two-dimensional structure, and achieve the reaction Simple and easy operation, high reaction yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole (tatrz)
[0031] The molar ratio of 9,10-dibromoanthracene: triazole: potassium carbonate: copper oxide is 2:10:30:1;
[0032] CuO (0.0398 mg, 0.5 mmol), potassium carbonate (2.0731 g, 15 mmol), triazole (0.345 mg, 5 mmol), and 9,10-Dibromoanthracene (0.3360 g, 1 mmol), 20 mL DMF. Start stirring at 100 o C, reacted for 24 hours. After the reaction, the reaction solution was lowered to room temperature, filtered, and 100 mL of water was added to the filtrate, a large amount of precipitate was precipitated, filtered with suction, and the filter cake was collected, with a yield of 60%.
Embodiment 2
[0034] Complex 1 preparation of
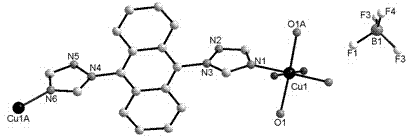
[0035] Cu(BF 4 ) 2 and 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole (tatrz) in a molar ratio of 1: 1;
[0036] tatrz (0.0625 g, 0.2 mmol) and Cu(BF 4 ) 2(0.0690 g, 0.2 mmol) in H 2 O (6 mL) and CH 3 OH (4 mL) in a mixed solvent at room temperature for half an hour and then filtered, and the filtrate was volatilized at room temperature for two weeks to obtain green rod-shaped crystals suitable for X-ray single crystal diffraction analysis. Yield: 78%. Elemental Analysis (%) C 36 h 28 B 2 CuF 8 N 12 o 2 : C 48.16, H 3.14, N 18.72; Found: C 48.08, H 3.10, N 18.68.
Embodiment 3
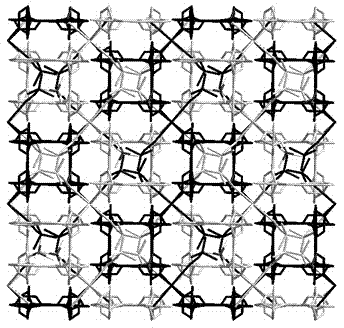
[0038] Selective Ion Exchange Properties of Complexes
[0039] in the complex 1 (0.4672 g, 0.5 mmol) and NaClO 4 (0.7023 g, 5 mmol) in a mixed solvent of water and methanol (10 mL, v:v = 5:5) was stirred for 6 hours, and green rod-shaped crystals suitable for X-ray single crystal diffraction analysis were obtained 1a , Yield: 25%. Elemental Analysis (%) C 36 h 41.13 Cl 2 CuN 12 o 15.56 : C 42.17, H 4.04, N 16.39; Found: C 47.08, H 4.02, N 16.38.
[0040] The crystal structure was determined using an APEX II CCD single crystal diffractometer, using graphite monochromatized Mokα rays (λ = 0.71073 ?) as the incident radiation. ω -2 θ Diffraction points are collected by scanning, and the unit cell parameters are obtained by least square method correction. The crystal structure is solved by software from the difference Fourier electron density map, and corrected by Lorentz and polarization effects. All H atoms were synthesized by difference Fourier transform a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com