A method for separating r/s-3-quinuclidinol by pre-column derivatization high performance liquid chromatography
A high-performance liquid chromatography, S-3-technology, applied in the field of resolution of R/S-3-quinuclidinol, to achieve improved peak shape, fast and sensitive detection, chromatographic peak shape and good symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
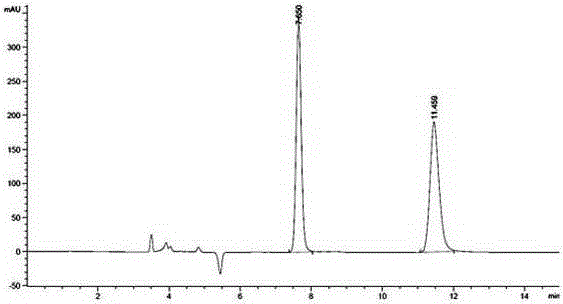
Embodiment 1
[0030] A method utilizing pre-column derivation high performance liquid chromatography to resolve R / S-3-quinuclidinol, specifically comprising the following steps:
[0031] (1) R / S-3-quinuclidinol is derivatized and esterified before the column to obtain R / S-3-quinuclidinol ester, and the obtained R / S-3-quinuclidinol ester, its The structural formula is as follows:
[0032]
[0033] where R is COC 6 h 5 ;
[0034] The pre-column derivatization esterification is about R / S-3-quinuclidinol and aromatic acid in ethyl acetate solvent, DMF is used as a catalyst for esterification to obtain R / S-3-quinuclidinol ester;
[0035] The amount of aromatic acid, R / S-3-quinuclidinol and catalyst used in the above-mentioned esterification is calculated in molar ratio, that is, aromatic acid: R / S-3-quinuclidinol: DMF is 1:1:0.1 ;
[0036] Wherein said aromatic acid is benzoic acid;
[0037] The above-mentioned pre-column derivative esterification reaction specifically comprises the ste...
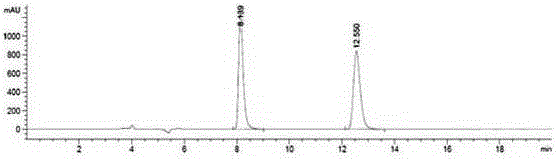
Embodiment 2
[0051] A method utilizing pre-column derivation high performance liquid chromatography to resolve R / S-3-quinuclidinol, specifically comprising the steps of:
[0052] (1) R / S-3-quinuclidinol is derivatized and esterified before the column to obtain R / S-3-quinuclidinol ester, and the obtained R / S-3-quinuclidinol ester, its The structural formula is as follows:
[0053]
[0054] where R is COC 10 h 7 ;
[0055] The pre-column derivatization esterification is about R / S-3-quinuclidinol and aromatic acid in ethyl acetate solvent, DMF is used as a catalyst for esterification to obtain R / S-3-quinuclidinol ester;
[0056] The amount of aromatic acid, R / S-3-quinuclidinol and catalyst used in the above-mentioned esterification is calculated in molar ratio, that is, aromatic acid: R / S-3-quinuclidinol: DMF is 1:1:0.1 ;
[0057] Wherein said aromatic acid is 1-naphthoic acid;
[0058] The above-mentioned pre-column derivative esterification reaction specifically comprises the steps...
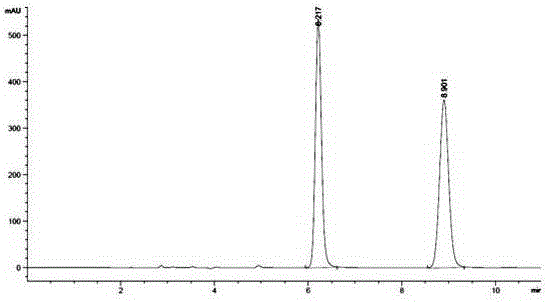
Embodiment 3
[0076] A method utilizing pre-column derivation high performance liquid chromatography to resolve R / S-3-quinuclidinol, specifically comprising the steps of:
[0077] (1) R / S-3-quinuclidinol is derivatized and esterified before the column to obtain R / S-3-quinuclidinol ester, and the obtained R / S-3-quinuclidinol ester, its The structural formula is as follows:
[0078]
[0079] where R is COC 6 h 4 OCH3 ;
[0080] The pre-column derivatization esterification is about R / S-3-quinuclidinol and aromatic acid in ethyl acetate solvent, DMF is used as a catalyst for esterification to obtain R / S-3-quinuclidinol ester;
[0081] The amount of aromatic acid, R / S-3-quinuclidinol and catalyst used in the above-mentioned esterification is calculated in molar ratio, that is, aromatic acid: R / S-3-quinuclidinol: DMF is 1:1:0.1 ;
[0082] Wherein said aromatic acid is p-methoxybenzoic acid;
[0083] The above-mentioned pre-column derivative esterification reaction specifically comprises ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com