Mucoadhesive nanoparticle delivery system
A nanoparticle, mucosal technology, applied in respiratory system diseases, urinary system diseases, cardiovascular system diseases, etc., can solve non-specific problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0249] In some embodiments, there is provided a nanoparticle composition for delivering a payload to a mucosal site, the nanoparticle comprising a plurality of amphiphilic macromolecules comprising: a hydrophobic moiety comprising polylactide, polyethylene Biocompatible polymers of lactide, poly(lactide-co-glycolide), poly(ε-caprolactone), or combinations thereof; hydrophilic moieties comprising polysaccharides, polynucleotides, polypeptides or A biocompatible polymer of its combination, the hydrophilic portion includes a plurality of functional moieties; and a mucosal targeting portion selected from phenylboronic acid (PBA) derivatives, thiol derivatives or acrylate derivatives, wherein the hydrophilic At least a portion of the functional moiety of the moiety is conjugated to a mucosal targeting moiety.
[0250] In some embodiments, there is provided a nanoparticle composition for delivering a payload to a mucosal site, the nanoparticle comprising a plurality of amphiphilic m...
Embodiment 1
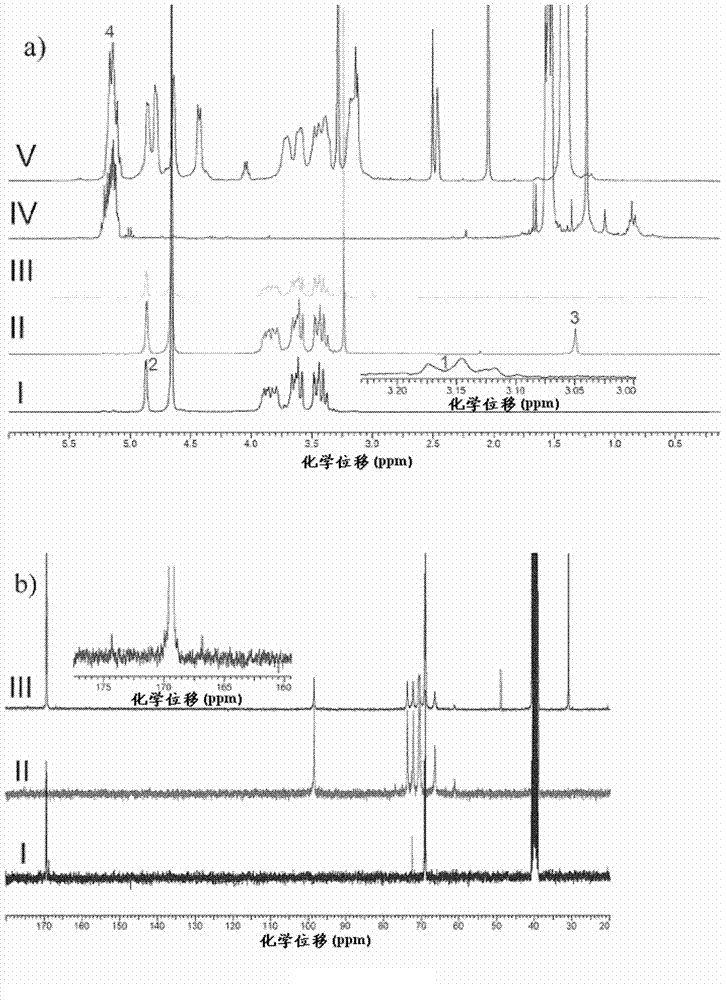
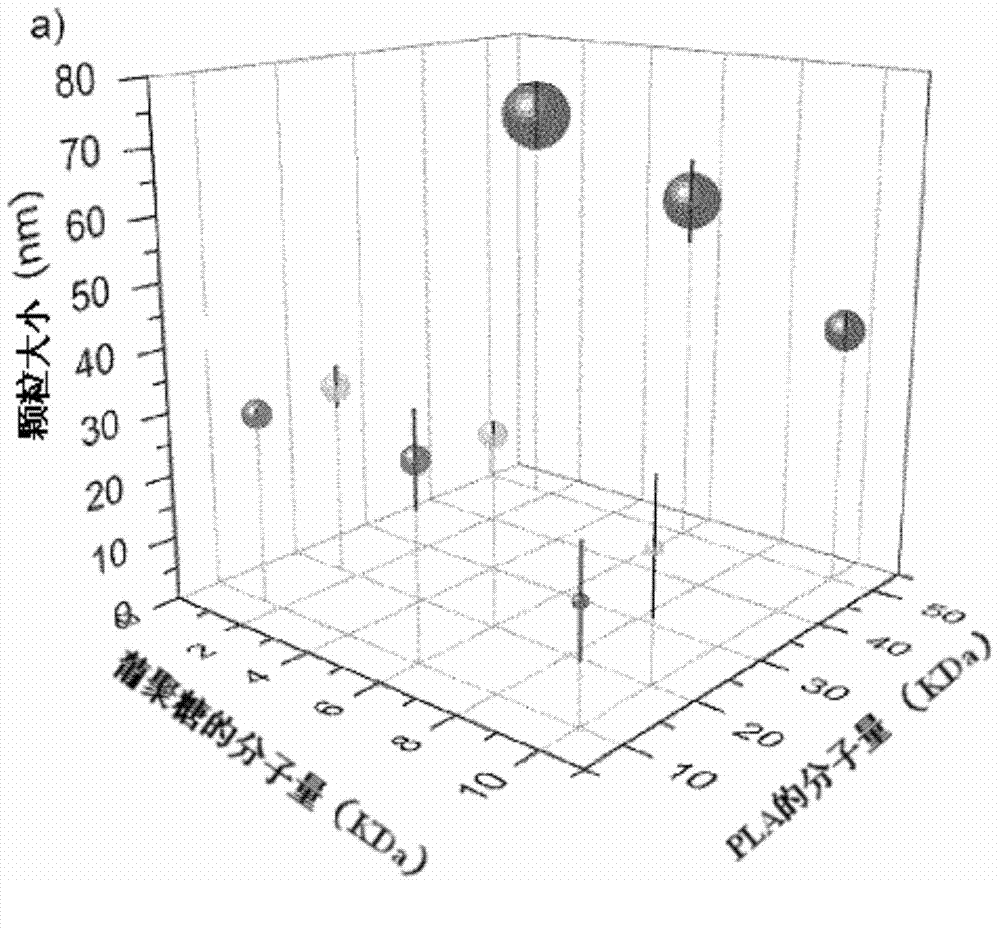
[0261] Synthesis and characterization of embodiment 1.Dex-b-PLA
[0262] 1.1 Materials
[0263] Acid-terminated poly(D,L-lactide) (PLA, M w ~10, 20 and 50kDa) and PLGA-PEG (PLGA M w ~40kDa, PEG M w ~6 kDa) were purchased from Lakeshore Biomaterials (Birmingham, AL, USA). PLA was purified by dissolving PLA in dimethylsulfoxide (DMSO) and precipitating in methanol to remove residual monomers. Dextran (Dex, M r ~1.5, 6, and 10 kDa), hydrochloric acid (HCl), triethylamine (TEA), N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC), and sodium cyanoborohydride (NaCNBH 3 ) were purchased from Sigma Aldrich (Oakville, ON, Canada) and used without further purification. N-Hydroxysulfosuccinimide (thio-NHS) and N-tert-butoxycarbonylethylenediamine were purchased from CNH Technologies (Massachusetts, USA). Deprotonate doxorubicin-HCl (MW=580 Da, Intatrade GmBH, Bitterfield, Germany) by adding TEA (2M equiv.) in aqueous doxorubicin-HCl solution and extract with dichloromethane (DCM...
Embodiment 2
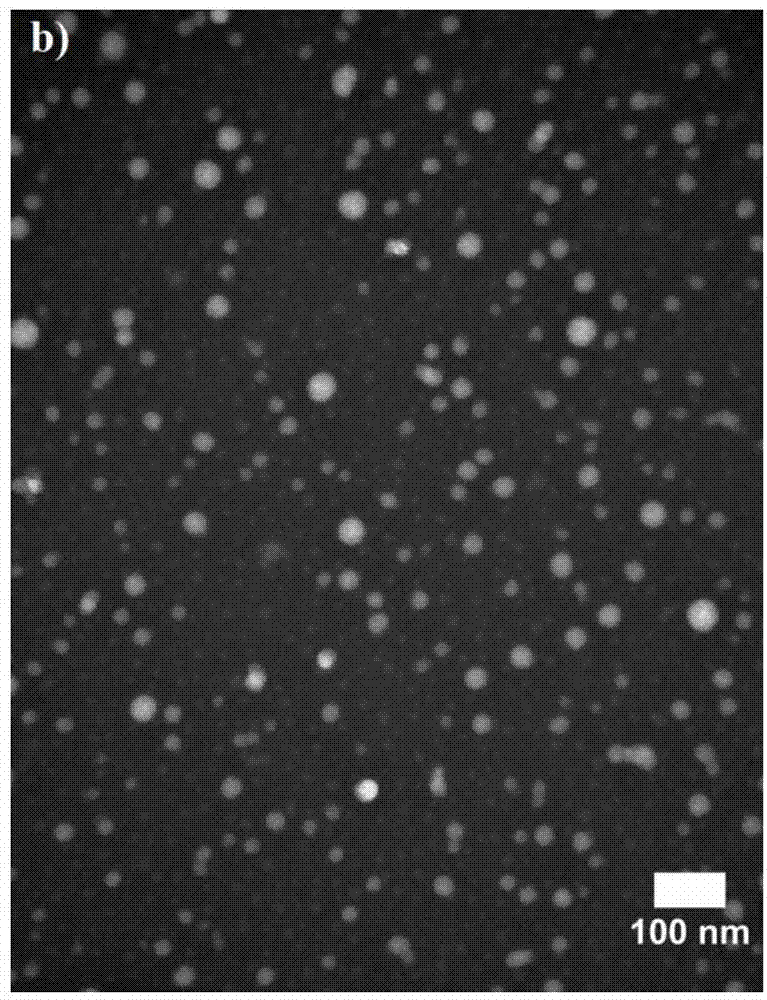
[0278] Example 2. Encapsulation and in vitro of doxorubicin in Dex-b-PLA NP by nanoprecipitation method
[0279] Encapsulation of doxorubicin in Dex-b-PLA NPs was accomplished using the nanoprecipitation method. Both Dex-b-PLA and doxorubicin were dissolved in DMSO (7 mg / mL Dex-b-PLA concentration, varying drug concentrations). 1 mL of the DMSO solution was added to 10 mL of water in a dropwise fashion with stirring, and stirring was continued for an additional 30 minutes. NPs in water were filtered through syringe filters (pore size = 200 nm) to remove drug aggregates, and then filtered through ultrafiltration filter tubes (MWCO = 10 kDa, Millipore) to remove any remaining free drug in suspension. Filtered NPs containing encapsulated doxorubicin were resuspended and diluted in DMSO. Therefore, the drug loading (wt %) in the polymer matrix was calculated by measuring the concentration of doxorubicin in the mixture by obtaining the absorbance of the solution at 480 nm using a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com