Bipolar polymer blue light host material, preparation method and applications thereof
A blue light host material and polymer technology, which can be used in chemical instruments and methods, semiconductor/solid-state device manufacturing, compounds of Group 5/15 elements of the periodic table, etc. Improved luminous efficiency and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
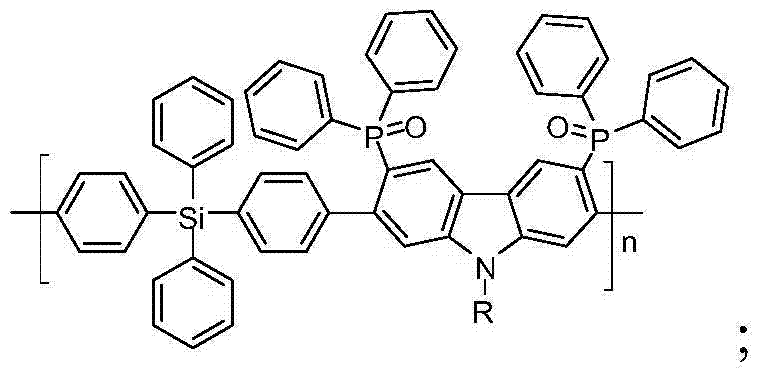
[0027] Example 1: The bipolar polymer blue light host material of this example, namely poly{bis(4-yl-phenyl)diphenylsilane-co-2,7-diyl-3,6-bis(di Phenylphosphineoxy)-9-n-hexyl-9H-carbazole} (P1) (where R is n-hexyl, n=45), its structural formula is as follows:
[0028]
[0029] The preparation steps of above-mentioned polymer are as follows:
[0030] The reaction formula is as follows:
[0031]
[0032] Under argon protection, bis(4-bromophenyl)diphenylsilane (99mg, 0.2mmol), 2,7-dibromo-3,6-bis(diphenylphosphineoxy)-9-n-hexyl -9H-carbazole (162mg, 0.2mmol) was added to a flask filled with 10ml of toluene solvent, and after fully dissolving, potassium carbonate (2mL, 2mol / L) solution was added to the flask, vacuumed to remove oxygen and filled with argon, Then bistriphenylphosphine palladium dichloride (5.6mg, 0.008mmol) was added; the flask was heated to 100°C for Suzuki coupling reaction for 48h. Subsequently, the polymerization reaction was stopped after cooling do...
Embodiment 2
[0035] Embodiment 2: The bipolar polymer blue light host material of this embodiment, that is, poly{bis(4-yl-phenyl)diphenylsilane-co-2,7-diyl-3,6-bis(di Phenylphosphineoxy)-9-n-eicosyl-9H-carbazole} (P2) (where R is n-eicosyl, n=98), its structural formula is as follows:
[0036]
[0037] The preparation steps of above-mentioned polymer are as follows:
[0038] The reaction formula is as follows:
[0039]
[0040] Under the protection of mixed gas of nitrogen and argon, bis(4-bromophenyl)diphenylsilane (150mg, 0.3mmol), 2,7-dibromo-3,6-bis(diphenylphosphineoxy)-9 - Add n-eicosyl-9H-carbazole (302mg, 0.3mmol) and 15mL of tetrahydrofuran into a 50mL two-necked bottle, fully dissolve, pass in a mixture of nitrogen and argon to exhaust the air for about 20min, and then place tetrahydrofuran Phenylphosphine palladium (4mg, 0.003mmol) was added, and after fully dissolved, sodium bicarbonate (3mL, 2mol / L) was added. Then, the mixed gas of nitrogen and argon was exhausted fo...
Embodiment 3
[0041] Embodiment 3: The bipolar polymer blue light host material of this embodiment, that is, poly{bis(4-yl-phenyl)diphenylsilane-co-2,7-diyl-3,6-bis(di Phenylphosphineoxy)-9-methyl-9H-carbazole} (P3) (where R is methyl, n=80), its structural formula is as follows:
[0042]
[0043] The preparation steps of above-mentioned polymer are as follows:
[0044] The reaction formula is as follows:
[0045]
[0046] Under nitrogen protection, bis(4-bromophenyl)diphenylsilane (150mg, 0.3mmol), 2,7-dibromo-3,6-bis(diphenylphosphineoxy)-9-methyl-9H -Carbazole (244mg, 0.33mmol), palladium acetate (3.5mg, 0.015mmol) and three (o-methylphenyl) phosphine (21mg, 0.06mmol) were added to 12mL of N,N-dimethylformamide After fully dissolving, add potassium carbonate (3mL, 2mol / L) solution into the flask, then blow nitrogen into the flask and exhaust the air for about 30min; heat the flask to 130°C for Suzuki coupling reaction for 12h. Subsequently, stop the polymerization reaction after c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com