A kind of sulpiride tablet and preparation method thereof
A technology for sulpiride tablets and prescriptions, which is applied in the field of sulpiride tablets and its preparation, can solve problems such as unsatisfactory dissolution rate of sulpiride tablets, and achieve the effects of increasing bioavailability, improving dissolution rate, and good compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) The preparation of solid dispersion carrier, PEG4000 300g, PVPk30 20g are joined in the 95% (ml / ml) ethanol of formula quantity 2 / 3, heat and stir until PEG4000 and PVPk30 dissolve completely, get adjuvant solution; 1000g sulpiride is dissolved in the 95% (ml / ml) ethanol of recipe quantity (remainder) 1 / 3 to obtain main drug solution; Described adjuvant solution and described main drug solution are mixed uniformly to obtain mixed solution; The solution was vacuum-dried for 12 hours (vacuum degree less than 10Pa), the drying temperature was 35°C, and the moisture content was controlled to be less than 1% (mass percentage);
[0018] (2) Tablet preparation, pulverize the material obtained in step (1), and pass through a 100-mesh standard sieve to obtain a solid dispersion powder; put the gained solid dispersion powder, 200 g of lactose, and 300 g of microcrystalline cellulose into a granulator, and dry mix 5min, add 40% (ml / ml) ethanol of 150g as binding agent, set the...
Embodiment 2
[0023] (1) The preparation of solid dispersion carrier, PEG4000 300g, PVPk30 30g are joined in the 95% (ml / ml) ethanol of formula quantity 2 / 3, heat and stir until PEG4000 and PVPk30 dissolve completely, get adjuvant solution; 1000g sulpiride is dissolved in the 95% (ml / ml) ethanol of recipe quantity (remainder) 1 / 3 to obtain main drug solution; Described adjuvant solution and described main drug solution are mixed uniformly to obtain mixed solution; The solution was vacuum-dried for 24 hours (vacuum degree less than 10Pa), the drying temperature was 55°C, and the moisture content was controlled to be less than 1% (mass percentage);
[0024] (2) Tablet preparation, pulverize the material obtained in step (1), and pass through a 120-mesh standard sieve to obtain a solid dispersion powder; put the gained solid dispersion powder, 200 g of lactose, and 300 g of microcrystalline cellulose into a granulator, and dry mix 10min, add the 45% (ml / ml) ethanol of 200g as binding agent, se...
Embodiment 3
[0026] Tablet Quality Check
[0027] 1. Appearance: The surface of the sulpiride tablet prepared in Examples 1 and 2 is smooth, and it is brighter and whiter than the tablet prepared by traditional techniques.
[0028] 2. Dissolution rate:
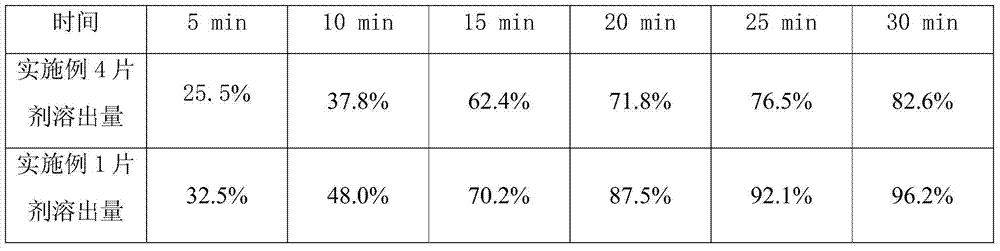
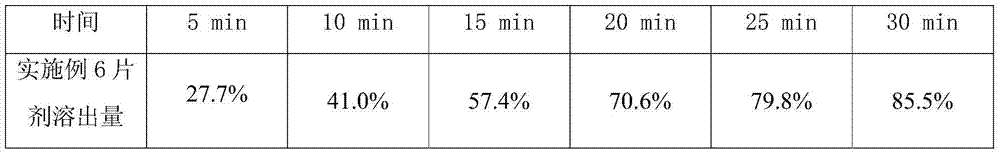
[0029] Embodiment 1,2 gained sulpiride tablet respectively gets 6, checks dissolution rate according to two appendix XC of Chinese Pharmacopoeia 2010 edition, and the dissolution rate of the tablet prepared in embodiment 1,2 is all more than 85% in 30 minutes (according to labeling meter).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com