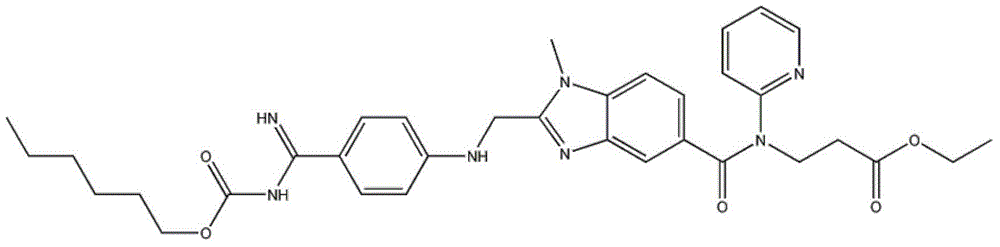
Pradaxa dropping pill and preparation method thereof
A technology of dabigatran etexilate and dropping pills, which is applied in the field of pharmaceutical preparations and its preparation, can solve the problems of slow onset of action, heavy taste, and large dosage, and achieve faster dissolution and absorption, easy absorption, and drug dispersion uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention provides a dabigatran etexilate dripping pill and a preparation method thereof. In the method, taking dabigatran etexilate as the main raw material, according to a certain ratio, adding substrates such as polyethylene glycol, after passing through specific processes and equipment Prepared by processing. details as follows:
[0022] (1) Prescription: dabigatran etexilate + matrix
[0023] Base: Contains polyethylene glycol (1500, 2000, 4000, 6000, 8000, 10000, 20000), polyoxyl 40 stearate, beta cyclodextrin, poloxamer, sodium carboxymethyl starch, One or more components of stearic acid, sodium stearate, glycerin gelatin, glyceryl monostearate, shellac, polyoxyethylene monostearate, polyether.
[0024] The weight ratio of dabigatran etexilate and substrate is 1: (1-60).
[0025] (2) Preparation process: the specific implementation steps are as follows:
[0026] The first step is to mix the dabigatran etexilate with the matrix according to a certain ratio...
Embodiment 1
[0042] Prescription: Dabigatran etexilate 0.5g; PEG4000 5g;
[0043] PEG8000 25g; 1000 capsules in total.
[0044] Preparation method: mix dabigatran etexilate, PEG4000 and PEG8000 evenly, heat in a water bath to make it melt completely, pour the molten liquid into the material funnel of the dripping pill making machine, keep it warm at 80°C, select the drip nozzle, and drop it into Put it in simethicone oil at -5°C, after forming, filter, separate, and wipe clean to get it.
Embodiment 2
[0046] Prescription: Dabigatran etexilate 1g; PEG1500 5g;
[0047] PEG6000 25g; 1000 capsules in total.
[0048] Preparation method: Mix dabigatran etexilate, PEG1500, and PEG6000 evenly, heat in a water bath to make it completely melt, pour the molten liquid into the material funnel of the dripping pill making machine, keep it warm at 80°C, select the drip nozzle, and drop it into Put it in simethicone oil at -5°C, after forming, filter, separate, and wipe clean to get it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com