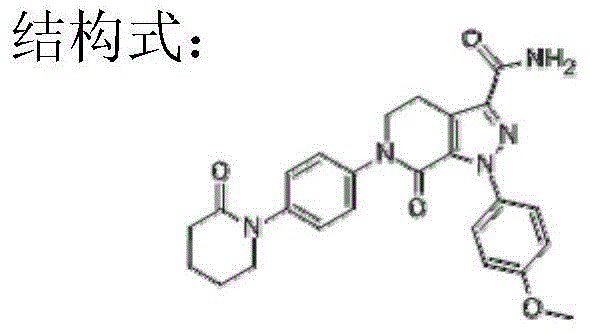
Apixaban dropping pill and preparation method thereof
A technology for apixaban and dropping pills is applied in the field of pharmaceutical preparations and their preparation, which can solve the problems of heavy and difficult taste, large dosage, slow onset of effect, etc., and achieves easy absorption, uniform drug dispersion, dissolution and absorption accelerated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention provides an apixaban drop pill and a preparation method thereof. In the method, apixaban is used as the main raw material, and polyethylene glycol and other substrates are added in a certain proportion, and prepared through specific processes and equipment. made. details as follows:
[0022] (1) Prescription: Apixaban + matrix
[0023] Base: Contains polyethylene glycol (1500, 2000, 4000, 6000, 8000, 10000, 20000), polyoxyl 40 stearate, beta cyclodextrin, poloxamer, sodium carboxymethyl starch, One or more components of stearic acid, sodium stearate, glycerin gelatin, glyceryl monostearate, shellac, polyoxyethylene monostearate, polyether.
[0024] The weight ratio of apixaban to base is 1:(1-60).
[0025] (2) Preparation process: the specific implementation steps are as follows:
[0026] The first step is to mix apixaban with the matrix according to a certain ratio.
[0027] The second step is to use water bath, oil bath or other heating methods to h...
Embodiment 1
[0041] Prescription: Apixaban 0.5g; PEG4000 5g;
[0042] PEG8000 25g; 1000 capsules in total.
[0043] Preparation method: Mix apixaban, PEG4000, and PEG8000 evenly, heat in a water bath to make it completely melt, pour the molten liquid into the material funnel of the dripping pill making machine, keep it warm at 80°C, select the drip nozzle, and drop it into the In simethicone oil at -5°C, after molding, filter, separate, and wipe clean to obtain.
Embodiment 2
[0045] Prescription: Apixaban 1g; PEG1500 5g;
[0046] PEG6000 25g; 1000 capsules in total.
[0047] Preparation method: Mix apixaban, PEG1500, and PEG6000 evenly, heat in a water bath to melt completely, pour the molten liquid into the material funnel of the dripping pill making machine, keep it warm at 80°C, select the drip nozzle, and drop it into the In simethicone oil at -5°C, after molding, filter, separate, and wipe clean to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com