Application of plectasin in inhibiting streptococcus agalactiae
A technology of lectin and Streptococcus lactis, which can be used in antibacterial drugs, medical preparations containing active ingredients, peptide/protein ingredients, etc., and can solve unknown problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation method of embodiment 1 plectasin protein
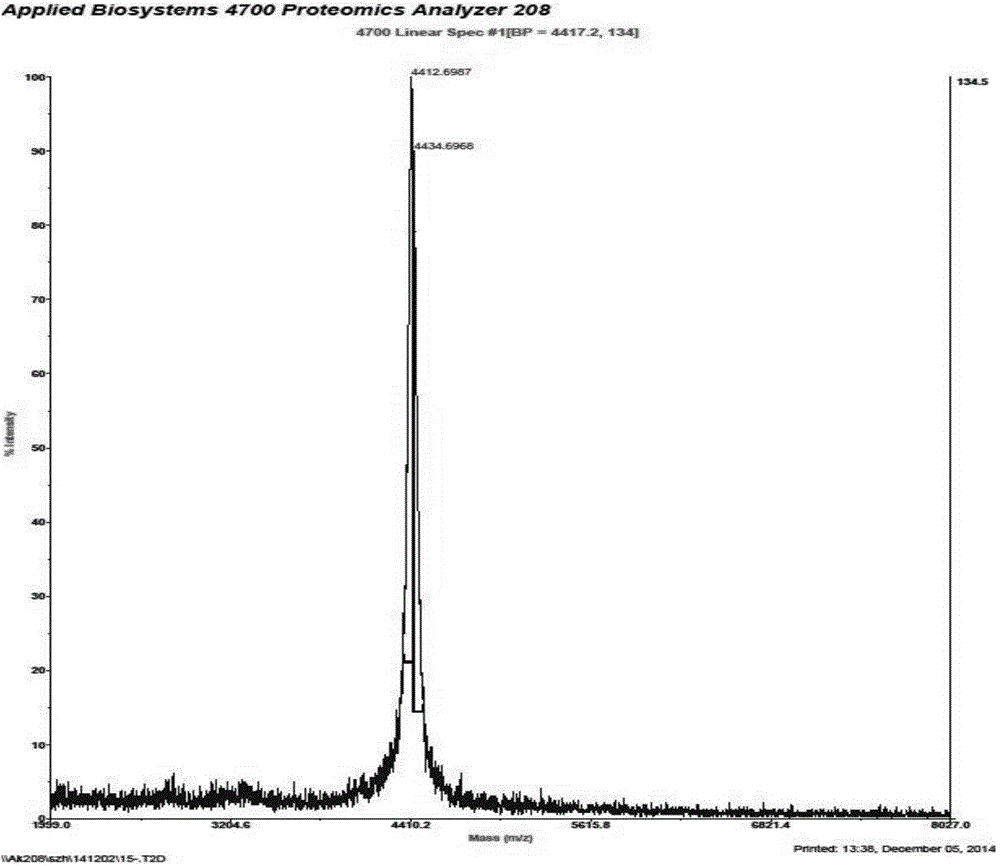
[0018] In this example, a method of exogenously expressing the target protein plectasin using Lactococcus lactis NZ9700 as the host was used to prepare high-purity plectasiacin.
[0019] 1. Construction of recombinant strains
[0020] The nucleotide sequence of the Usp45 protein signal peptide was cloned from Lactococcus lactis MG1363, and the Usp45 gene fragment was connected with the shuttle plasmid pMG36e to form plasmid pGMN1; the plasmid pGMN1 was connected with the Pnis-MCS segment gene amplified from the lactic acid bacteria plasmid pNZ8408, Construction of plasmid pGMN2; artificially synthesized lactic acid bacteria codon-biased plectasin gene fragment, the optimized plectasin base sequence is: GGT TTT GGT TGT AAT GGT CCA TGG GAT GAA GAT GAT ATG CAA TGT CAT AAT CAT TGT AAA TCA ATT AAA GGT TAT AAA GGT GGT TAT TGT GCT AAA GGT GGT TTT GTT TGT AAA TGT TAT (SEQ ID NO: 1), the optimized Plectasin gene fra...
Embodiment 2
[0033] Example 2 Inhibitory effect of plectasin protein on streptococcus agalactiae
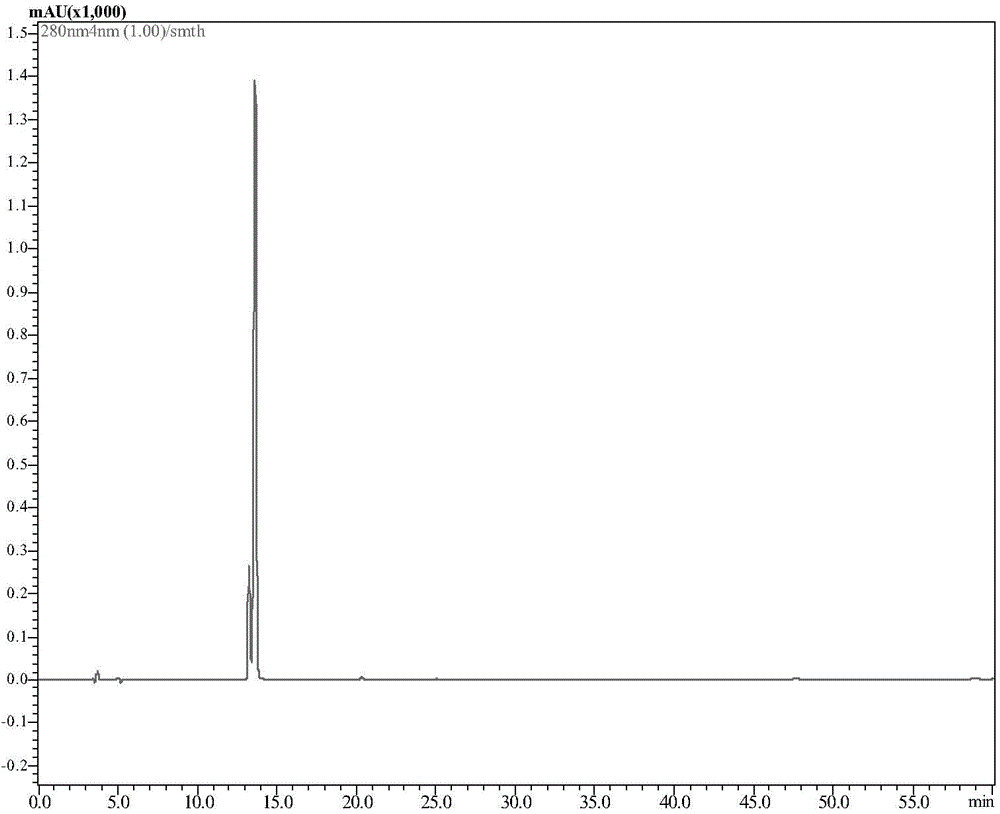
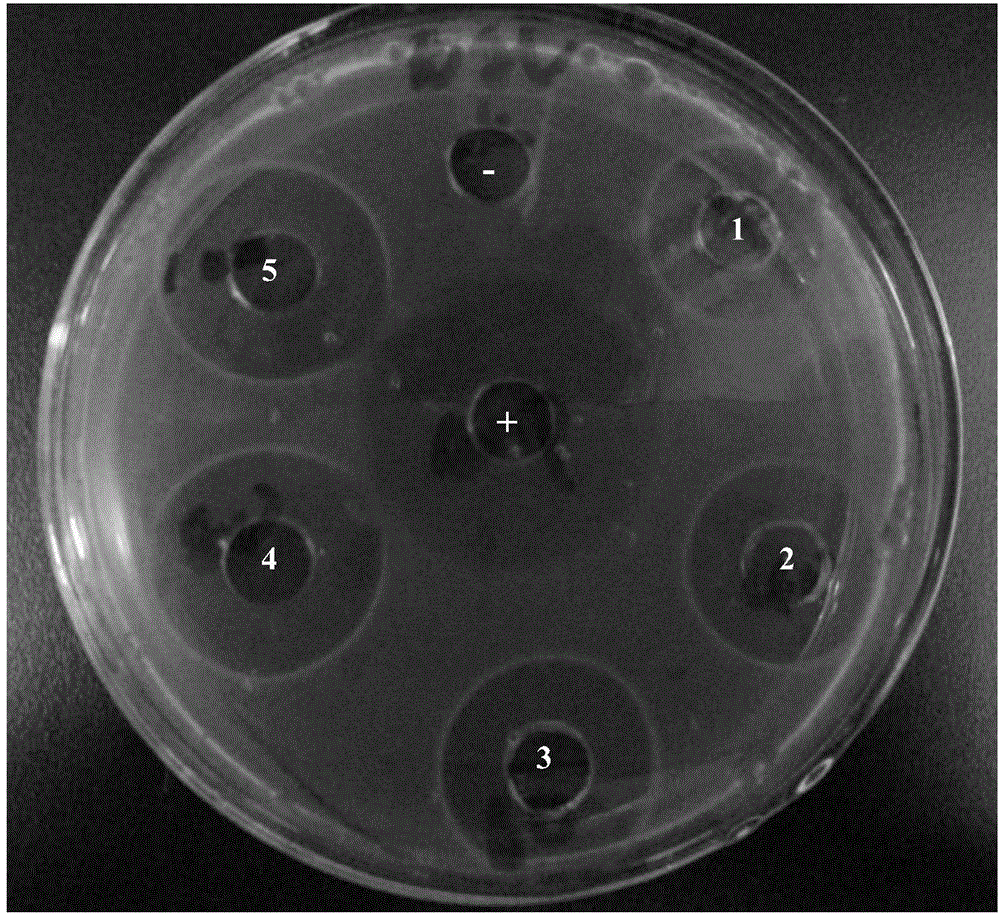
[0034]Take the above-mentioned mycelia protein purified by high-performance liquid chromatography, make it into a concentration of 1 μg / μL, and place the sample on the agar plate containing Streptococcus agalactiae; at the same time, the plate also contains ampicillin Amp and sterile water. Serve as positive and negative controls respectively; culture overnight at 37°C and observe the results.
[0035] The result is as image 3 As shown, there were obvious inhibition zones in all points where there were different volumes of mycelia solution and ampicillin, and the places where there was sterile water also listed the inhibition circles, indicating that mycelia had the ability to inhibit Streptococcus agalactiae effect.
Embodiment 3
[0036] Example 3 The minimum inhibitory concentration MIC test of Plectasia to Streptococcus agalactiae
[0037] The Streptococcus agalactiae liquid is adjusted with physiological saline to detect at a wavelength of 625nm, and the absorption value is 0.09-0.10, then diluted with sterilized broth in different proportions, and shaken evenly. Add different amounts of the above-mentioned purified plectasin or Amp into each test tube, so that the concentration of plectasin in each sample test tube is 1000 μg / mL, 100 μg / mL, 10 μg / mL, 1 μg / mL, 0.5μg / mL ; The concentration of Amp in each sample tube is 500μg / ml, 250μg / ml, 125μg / ml, 62μg / ml, 31μg / ml, 16μg / ml, 8μg / ml, 4μg / ml, 2μg / ml, 1μg / ml , 0.5 μg / ml, 0.25 μg / ml, 0.125 μg / ml, 0.062 μg / ml, 0.031 μg / ml. Then place it under the condition of 37° C. and incubate for 12 hours.
[0038] The test results showed that the MIC of Plectasia to Streptococcus agalactiae was 1 μg / mL of the purified Plectasia protein, and the MIC of Amp against ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com