An electrochemical measurement method
A measurement method and electrochemical technology, applied in the field of electrochemical measurement, can solve the problems of complex manufacturing of test strips, weak anti-interference ability, and lack of methods to effectively eliminate the negative interference of detection reduction current, so as to achieve a wide range of applications and anti-interference ability. Strong, eliminate negative interference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1. Reduce the positive interference of reducing interfering substances on the glucose electrochemical sensor with oxidation current as the detection signal.
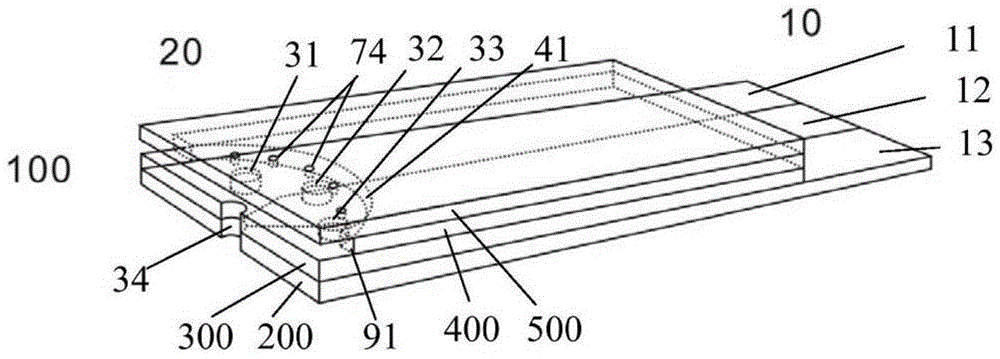
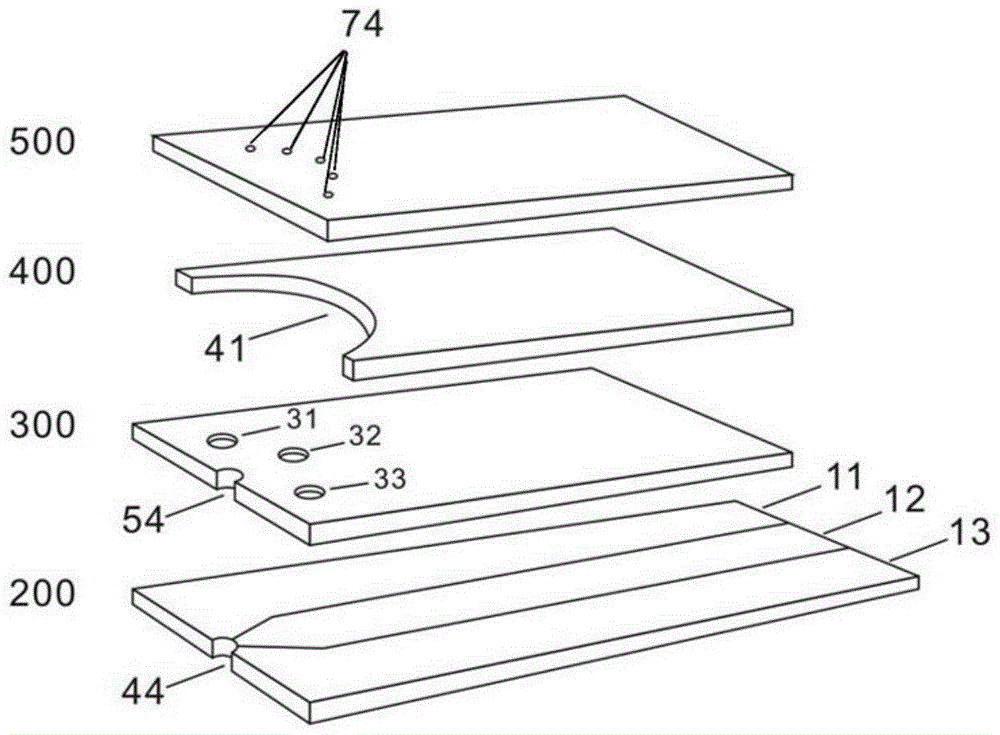
[0048] The first working electrode 11 is coated with glucose oxidase, oxidized electron mediator (potassium ferricyanide), polymer adhesion promoter (hydroxyethyl cellulose) and filler (lactose), and the second working electrode 13 and reference The specific electrode 12 is coated with an oxidized electron mediator (potassium ferricyanide), a polymer adhesion promoter (hydroxyethyl cellulose) and a filler (lactose). Link the electrochemical test strip with the potentiostatic measuring instrument (CHI electrochemical workstation, Shanghai Chenhua), and apply a positive voltage of 0.3V relative to the reference electrode 12 on the first working electrode 11 and the second working electrode 13 simultaneously, then The current I related to the glucose concentration is measured on the surface of the first wo...
Embodiment 2
[0055] Embodiment 2, reducing the negative interference of reducing interfering substances on the glucose electrochemical sensor with reducing current as the detection signal.
[0056] The structure of the electrochemical test strip used in Example 2 is the same as that in Example 1.
[0057] The first working electrode 11 is coated with glucose oxidase, peroxidase, reduced type electron mediator (potassium ferrocyanide), polymer adhesion promoter (hydroxyethyl cellulose) and filler (lactose), The second working electrode 13 is coated with novel ascorbate oxidase, peroxidase, reduced electron mediator (potassium ferrocyanide) and macromolecule adhesion promoter (hydroxyethyl cellulose) that catalyze ascorbic acid to produce hydrogen peroxide and filled with agent (lactose), and the reference electrode 12 is coated with a reduced electron mediator (potassium ferrocyanide), a polymer adhesion promoter (hydroxyethyl cellulose) and a filler (lactose). The electrochemical test str...
Embodiment 3
[0064] Embodiment 3, reducing the negative interference of reducing interfering substances to the blood lipid electrochemical sensor with reducing current as the detection signal
[0065] The structure of the electrochemical test strip used in embodiment 3 is the same as that in embodiment 1.
[0066] The first working electrode 11 is coated with cholesterol esterase, cholesterol oxidase, peroxidase, reduced electron mediator (potassium ferrocyanide), polymer adhesion promoter (hydroxyethyl cellulose) and filler ( lactose), the second working electrode 13 is coated with novel ascorbate oxidase, peroxidase, reduced electron mediator (potassium ferrocyanide) and polymer adhesion promoter (hydroxyethyl cellulose) that catalyze ascorbic acid to produce hydrogen peroxide ) and a filler (lactose), the reference electrode 12 is coated with a reduced electron mediator (potassium ferrocyanide), a polymer adhesion promoter (hydroxyethyl cellulose) and a filler (lactose). The electroche...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com