Polypeptide-calcitonin supramolecular aggregate with slow-release performance and preparation method thereof
A technology of supramolecular aggregates and sustained release performance, applied in the field of biomedicine, can solve the problems of high injection frequency, patient pain, short half-life, etc., and achieve the effects of good biocompatibility, guaranteed biological activity, and mild process conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
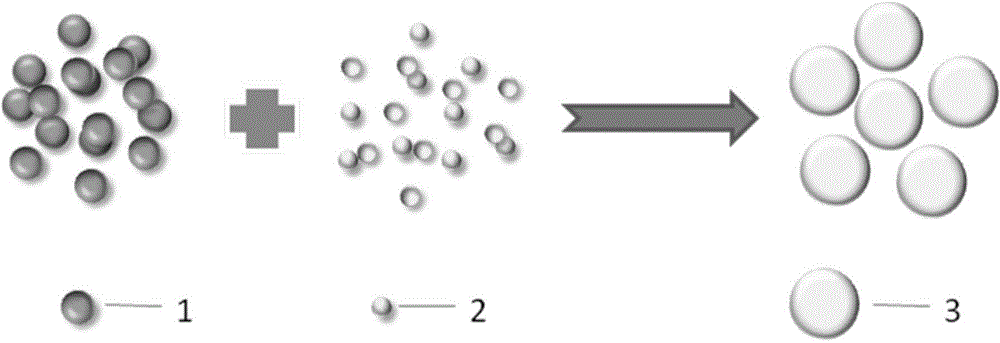
[0039] figure 1It is a schematic diagram of the formation process of the polypeptide-calcitonin supramolecular aggregate with slow-release performance described in the present invention, and the process steps of this embodiment are as follows:
[0040] (1) Add 8 g of salmon calcitonin and 4 g of amphiphilic polypeptide with amino acid sequence Asp-Phe (aspartic acid-phenylalanine) into a 50 mL round-bottomed flask equipped with a magnetic stir bar, and then add Add 20 mL of sodium phosphate buffer solution with pH=7.0 to form a mixed solution, the concentration of the salmon calcitonin in the mixed solution is 400 mg / mL, and the concentration of the amphiphilic polypeptide in the mixed solution is 200 mg / mL;
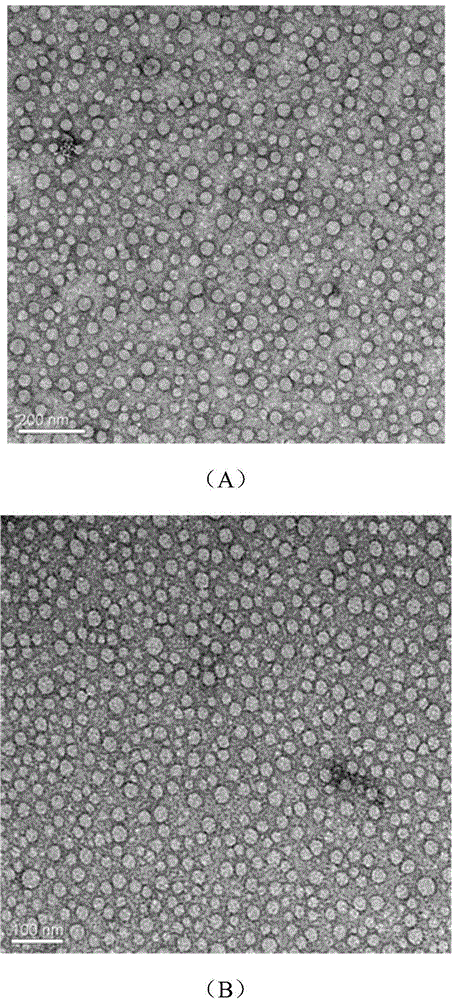
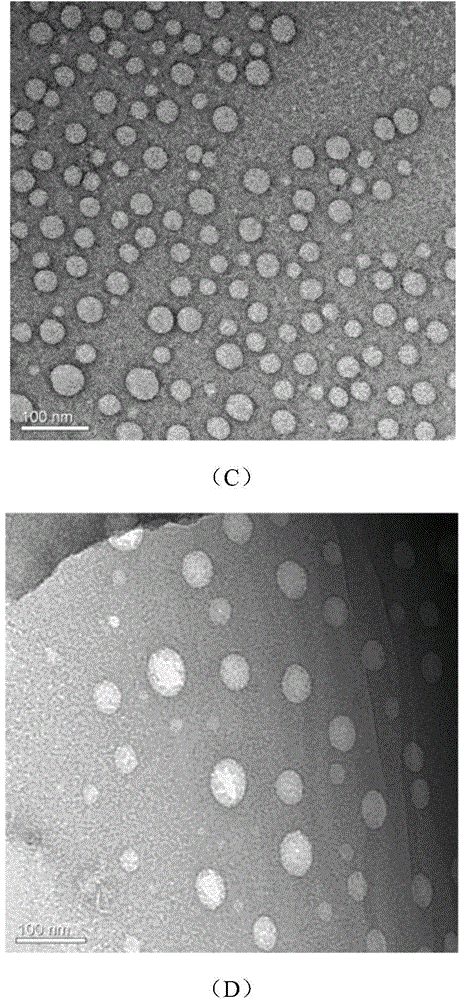
[0041] (2) Put the round-bottomed flask containing the mixed solution into a water bath on a constant temperature magnetic stirrer, the temperature of the water bath is 60°C, and then stir at a speed of 180r / min for 0.5h. After the stirring time expires, the supramolecul...
Embodiment 2
[0045] figure 1 It is a schematic diagram of the formation process of the polypeptide-calcitonin supramolecular aggregate with slow-release performance described in the present invention, and the process steps of this embodiment are as follows:
[0046] (1) Add 680 mg of salmon calcitonin and 1.94 g of the amphiphilic polypeptide whose amino acid sequence is shown in SEQ ID No: 5 in the sequence listing to a 50 mL round bottom flask equipped with a magnetic stirrer, and then add pH = 20 mL of sodium phosphate buffer solution of 5.0 forms a mixed solution, the concentration of the salmon calcitonin in the mixed solution is 34 mg / mL, and the concentration of the amphiphilic polypeptide in the mixed solution is 97 mg / mL;
[0047] (2) Put the round-bottomed flask containing the mixed liquid into a water bath on a constant temperature magnetic stirrer. The temperature of the water bath is 5°C, and then stir at a speed of 2000r / min for 1h. After the stirring time expires, supramolec...
Embodiment 3
[0051] figure 1 It is a schematic diagram of the formation process of the polypeptide-calcitonin supramolecular aggregate with slow-release performance described in the present invention, and the process steps of this embodiment are as follows:
[0052] (1) Add 0.2 g of salmon calcitonin and 0.2 g of the amphiphilic polypeptide whose amino acid sequence is shown in SEQ ID No: 1 in the sequence listing to a 50 mL round bottom flask equipped with a magnetic stirrer, and then add pH =8.0 sodium phosphate buffer 20mL forms a mixed solution, the concentration of the salmon calcitonin in the mixed solution is 10mg / mL, and the concentration of the amphiphilic polypeptide in the mixed solution is 10mg / mL;
[0053] (2) Put the round-bottomed flask containing the mixed liquid into a water bath on a constant temperature magnetic stirrer, the temperature of the water bath is 25°C, and then stir at a speed of 180r / min for 24h. After the stirring time expires, supramolecular aggregation is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com