Rheum emodin complexes and preparation method and application thereof
A technology of emodin complexes and emodin, which is applied in the field of emodin complexes and their preparation, can solve problems such as anti-cancer research of emodin metal complexes that have not been seen, and achieve improved anti-cancer, strong inhibitory effect, and anti-oxidation Active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
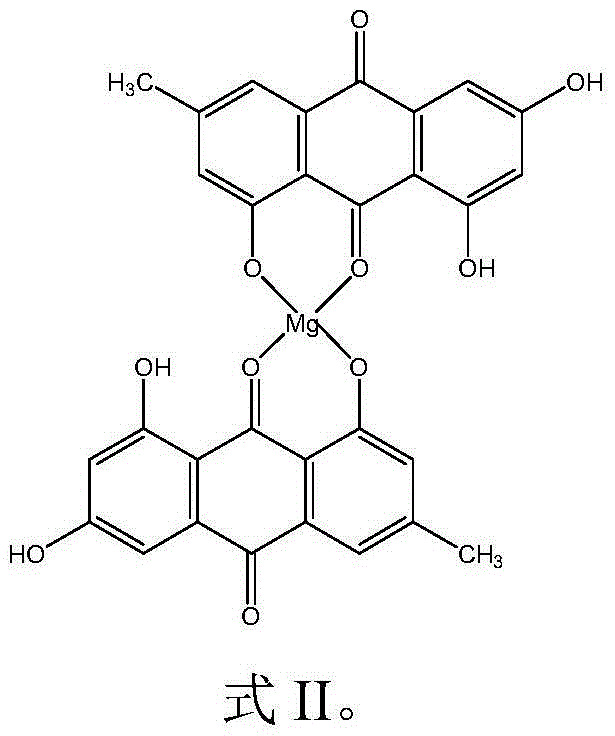
[0042] The preparation of embodiment 1 emodin magnesium (Ⅱ) complex
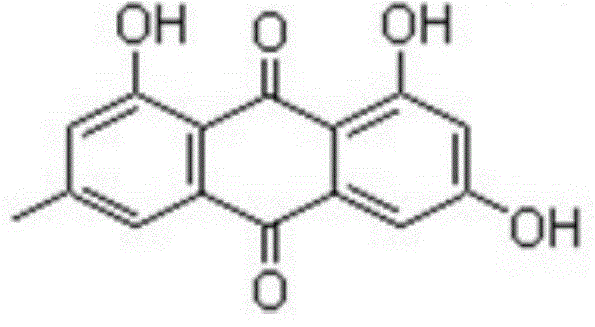
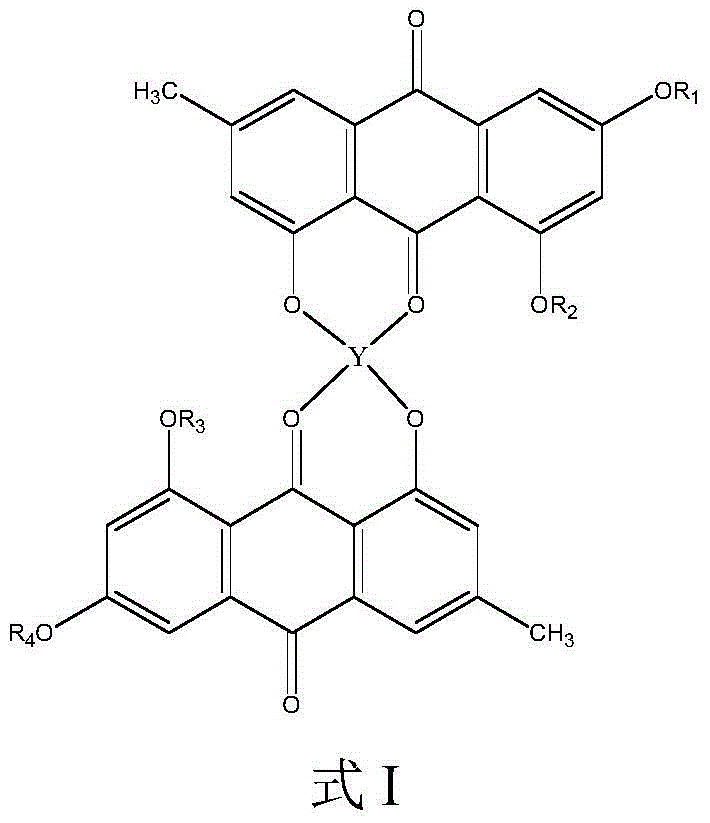
[0043] Weigh 1mmol emodin (270mg) and dissolve it in 60ml absolute ethanol, stir electromagnetically at room temperature, and obtain a yellow clear solution after most of the ligands are dissolved, add 0.5mmol (107.23mg) C 4 h 6 o 4 Mg·4H 2 O (magnesium acetate) in 15 mL of absolute ethanol solution, add ammonia water in ethanol solution (1:1, V:V) dropwise, adjust the pH of the ligand solution to 8-10, and continue to stir the reaction at a reaction temperature of 40°C After 12 hours, the solution was left to stand overnight for vacuum suction filtration, and the precipitate was washed several times with absolute ethanol and diethyl ether, respectively, and then vacuum-dried for 72 hours to obtain a powdery solid product, namely emodin magnesium (II) complex.
Embodiment 2
[0044] The preparation of embodiment 2 emodin calcium complexes
[0045] According to the method of Example 1, with CaCl 2 ·6H 2 O (calcium chloride) instead of zinc acetate, the reaction that is emodin calcium (Ⅱ) complex.
Embodiment 3
[0046] The preparation of embodiment 3 emodin chromium complexes
[0047] According to the method of embodiment 1, with CrCl 3 ·6H 2 O (chromium chloride) replaces zinc acetate, and reacts to obtain emodin chromium (Ⅲ) complexes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com