Method for synthesizing amino-modified NIT nitroxide free radicals
A technology of nitroxide free radical and amino modification, which is applied in the direction of organic chemistry, can solve the problems of low yield and difficulty of nitroxide free radical, etc., and achieve the effect of multiple reaction types, low density and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Dissolve 4 grams of N-(2-acetaldehyde)phthalimide and 3 grams of N,N'-dihydroxy-2,3-dimethyl-2,3-butanediamine in 70mL 0.1 mL of formic acid was added dropwise into 60% ethanol aqueous solution, stirred and reacted at 25°C for 24 hours, suction filtered and washed to obtain 4.2 g of white solid product A with a yield of 65%.
[0032] 2. Dissolve 1 gram of compound A in 50 mL of ethanol, add 0.1 gram of hydrazine hydrate, react at 60°C for 2 hours, cool to room temperature, filter with suction, remove the solvent to obtain a yellow oily liquid, dissolve in dichloromethane, and crystallize to obtain white Solid B 0.5 g, yield 83%.
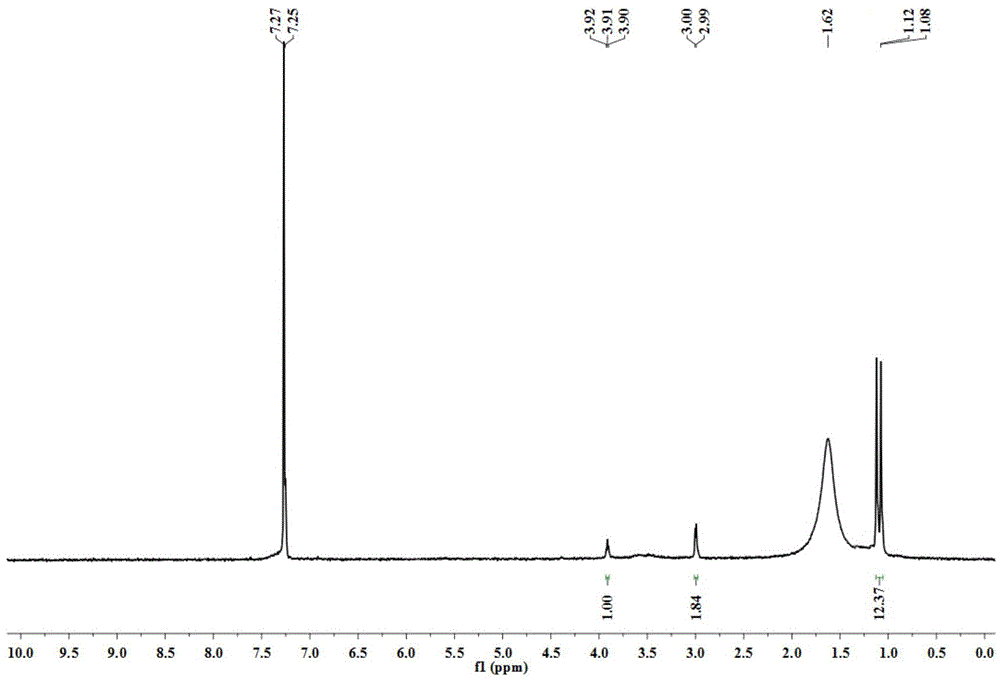
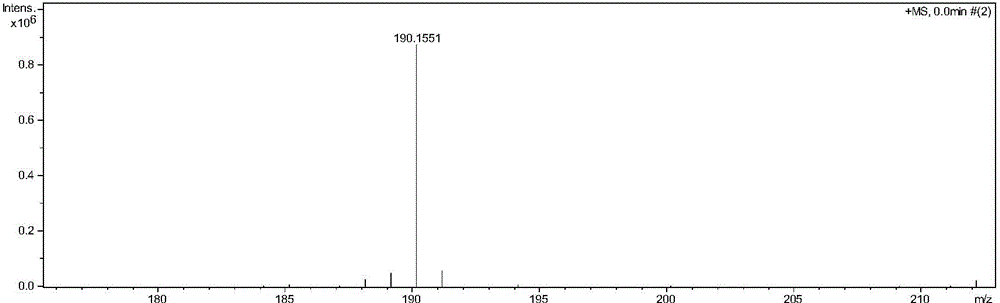
[0033] ESI-MS (m / z) = 190.1551. 1 H-NMR (300MHz, ppm, CDCl 3 )δ: 3,91(t,1H), 2.99(d,2H), 1.12(s,6H), 1.08(s,6H).
[0034] 3. Dissolve 0.5 g of compound B in 15 mL of methanol, add 5 g of lead dioxide, stir at room temperature for 2 hours, filter, and remove the solvent to obtain 0.3 g of red solid C with a yield of 61%.
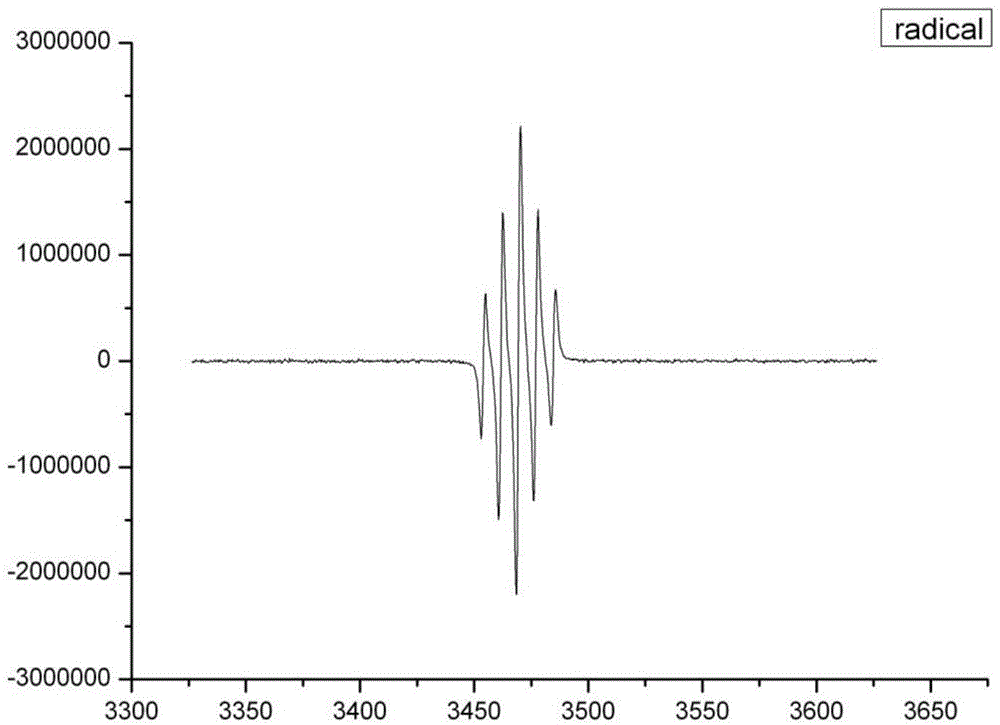
[0035] ESI-MS ...
Embodiment 21
[0036] Example 21. Dissolving 4 grams of N-(2-acetaldehyde)phthalimide and 3 grams of N,N'-dihydroxy-2,3-dimethyl-2,3-butanediamine 0.1 mL of formic acid was added dropwise into 70 mL of 40% ethanol aqueous solution, stirred and reacted at 20°C for 24 hours, suction filtered and washed to obtain 4.0 g of white solid product A with a yield of 62%.
[0037] 2. Dissolve 1 gram of compound A in 50 mL of ethanol, add 0.1 gram of hydrazine hydrate, react at 60°C for 2 hours, cool to room temperature, filter with suction, remove the solvent to obtain a yellow oily liquid, dissolve in dichloromethane, and crystallize to obtain white Solid B 0.5 g, yield 83%.
[0038] 3. Dissolve 0.5 g of compound B in 15 mL of methanol, add 5 g of lead dioxide, stir at room temperature for 2 hours, filter, and remove the solvent to obtain 0.3 g of red solid C with a yield of 61%.
Embodiment 3
[0040] 1. Dissolve 4 grams of N-(2-acetaldehyde)phthalimide and 3 grams of N,N'-dihydroxy-2,3-dimethyl-2,3-butanediamine in 70mL 0.1 mL of formic acid was added dropwise into 80% ethanol aqueous solution, stirred and reacted at 25°C for 30 hours, suction filtered and washed to obtain 4.1 g of white solid product A with a yield of 63%.
[0041] 2. Dissolve 1 gram of compound A in 50 mL of ethanol, add 0.1 gram of hydrazine hydrate, react at 60°C for 2 hours, cool to room temperature, filter with suction, remove the solvent to obtain a yellow oily liquid, dissolve in dichloromethane, and crystallize to obtain white Solid B 0.5 g, yield 83%.
[0042]3. Dissolve 0.5 g of compound B in 15 mL of methanol, add 5 g of lead dioxide, stir at room temperature for 2 hours, filter, and remove the solvent to obtain 0.3 g of red solid C with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com