Metal complex taking oxidized nantenine as ligand and synthetic method and applications thereof
A technology of metal complexes and nanthrophylline, applied in tin organic compounds, nickel organic compounds, chemical instruments and methods, etc., to achieve good potential medicinal value and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Synthesis of ONT-Mn (Ⅱ) complexes by solvothermal method
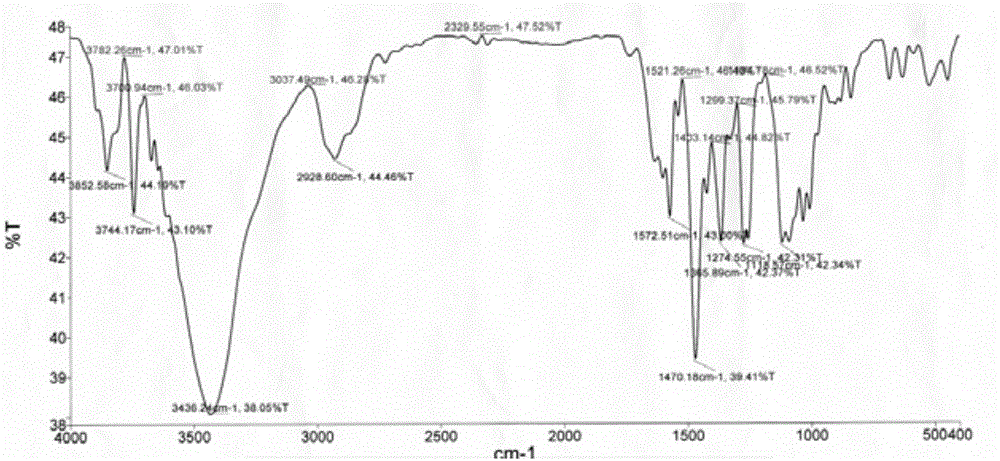
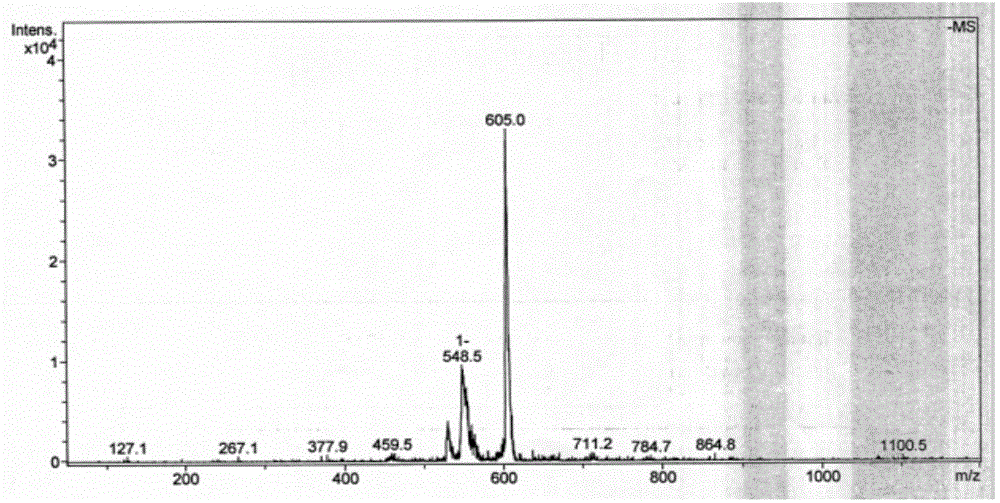
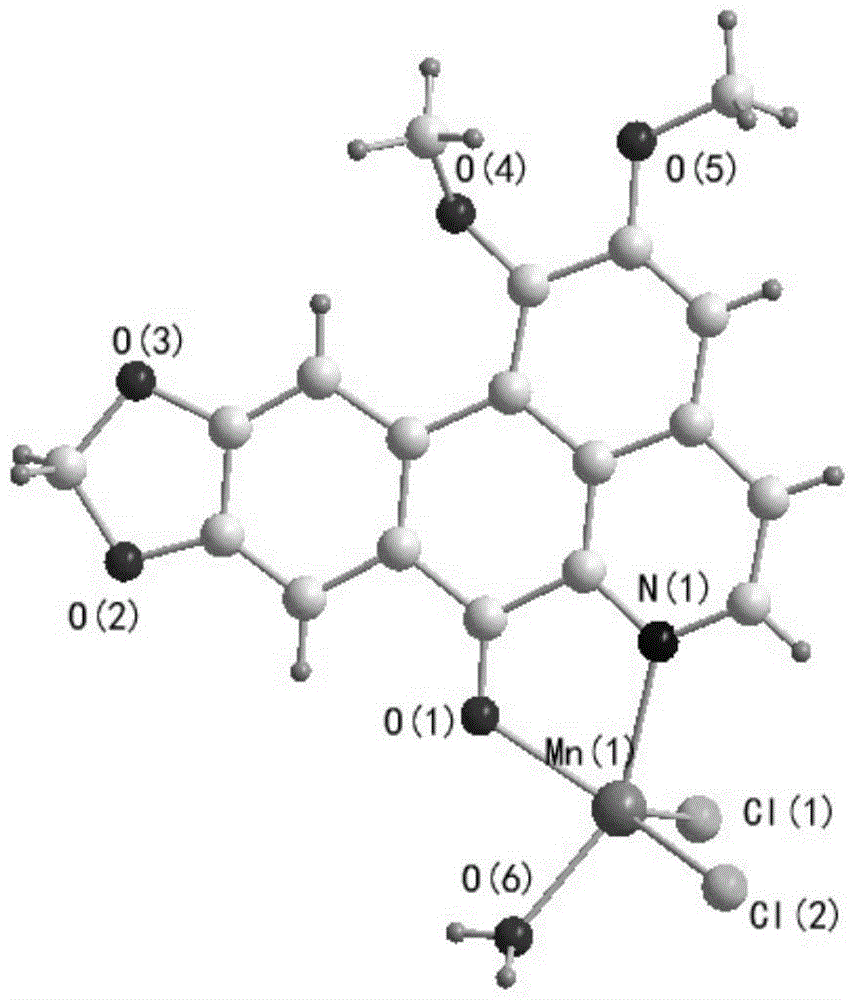
[0036] Weigh 0.1mmol ONT and dissolve it in 1mL DMSO, then weigh 0.1mmol MnCl 2 4H 2 O is dissolved in 0.5mL of methanol, and the two are added to a thick-walled borosilicate glass tube with an open end. Under the condition of liquid nitrogen freezing and vacuuming, the open end is melted and sealed, and then fully reacted at 80°C for 24 hours. Orange-red rhombic crystals were obtained, yield: 65%. The product is subjected to infrared spectroscopy (the spectrogram is as figure 1 shown), electrospray mass spectrometry (spectrum as shown in figure 2 shown) and X-ray single crystal diffraction (spectrum as image 3 Shown) analysis for structure determination, identified as the target complex [MnCl 2 (H 2 O)(ONT)].
Embodiment 2
[0037] Embodiment 2: Synthesis of ONT-Ni(Ⅱ) complex with solvothermal method
[0038] Weigh 0.3mmol NiCl 2 ·6H 2 O and 0.1mmol ONT were placed in a thick-walled borosilicate glass tube with an open end, and then 2.5mL of ethanol was added. Under the condition of liquid nitrogen freezing and vacuuming, the open end was melted and sealed, and then fully reacted at 100°C for 40h. The deep red crystalline solid product can be obtained, yield: 78%. The product is subjected to infrared spectroscopy (the spectrogram is as Figure 4 shown), electrospray mass spectrometry (spectrum as shown in Figure 5 shown) and X-ray single crystal diffraction (spectrum as Figure 6 Shown) analysis for structure determination, identified as the target complex [NiCl 2 (H 2 O) 2 (ONT)].
Embodiment 3
[0039] Embodiment 3: Synthesis of ONT-Ru(Ⅱ) complex by solvothermal method
[0040] Weigh 0.12mmol cis-[RuCl 2 (DMSO) 4] and 0.1 mmol ONT in a thick-walled borosilicate glass tube with an open end, and then add 1 mL of a mixed solvent of methanol and water (volume ratio of 3:1), and melt the open end under the condition of liquid nitrogen refrigeration and vacuum pumping. Sealed, and then fully reacted at 110°C for 12 hours to obtain dark green crystals with a yield of 39%. The product is subjected to infrared spectroscopy (the spectrogram is as Figure 7 shown), electrospray mass spectrometry (spectrum as shown in Figure 8 shown) and X-ray single crystal diffraction (spectrum as Figure 9 Shown) analysis for structure determination, identified as the target complex [RuCl 2 (DMSO) 2 (ONT)].
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com