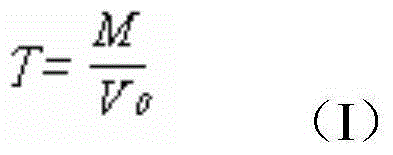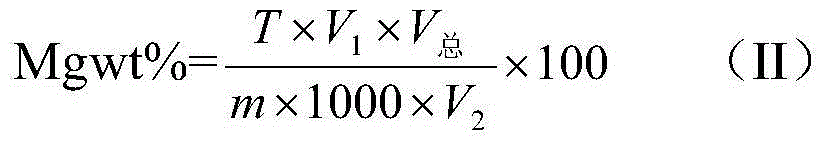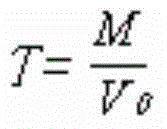Copper reagent separation-EDTA titration method for measuring magnesium content of aluminum alloy
A copper reagent, aluminum alloy technology, which is applied in material analysis by observing the influence of chemical indicators, and analysis by chemical reaction of materials, etc., can solve the large influence of iron determination, human and environmental damage, and large errors. and other problems, to achieve the effect of reducing operation steps, easy operation, accurate and stable measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 is to the detection of magnesium content in the aluminum alloy sample
[0042] 1. Determine the titer of magnesium
[0043] 1) Weigh the standard substance
[0044] Weigh a standard substance with similar magnesium content in the sample to be tested, its weight is shown in Table 1, and other components meet: iron content<0.0025wt%, silicon content<0.0010wt%, zinc content<0.0020wt%, manganese content< 0.0015wt%, copper content <0.0020wt%.
[0045] Table 1
[0046] Standard substance name
Magnesium content (wt%)
Sample weight (g)
aluminum alloy
4.48
0.5006
[0047] aluminum alloy
5.16
0.5031
[0048] 2) Dissolution of standard substances
[0049] Put the weighed standard substance in a 250ml beaker, add 6g of sodium hydroxide, 15mL of water, cover the watch glass and react violently, heat and dissolve on the electric furnace, add 150ml of hot water at 100°C (boiling), after cooling, compact t...
Embodiment 2
[0077] The aluminum alloy standard substances with magnesium contents of 5.16 wt % and 3.69 wt % were taken, and tested by the same method as in Example 1, and the results are shown in Table 4. It can be seen from the results in Table 4 that the error of the method of the present invention is ±0.10%, indicating that the present invention has good accuracy and precision.
[0078] Table 4
[0079]
[0080]
Embodiment 3
[0082] 1. The titer of pure magnesium
[0083] 1) Weigh 0.2000g~0.2200g of pure magnesium
[0084] table 5
[0085] Standard substance name
Sample weight (g)
Serving amount
pure magnesium
0.2005
5.00
pure magnesium
0.2148
5.00
[0086] Note: There is no impurity in this standard substance
[0087] 2) Dissolution of pure magnesium
[0088] Put the weighed pure magnesium into a 250ml beaker, add 6g of sodium hydroxide, 15mL of water, cover the watch glass and react violently, heat and dissolve on the electric furnace, transfer to a 200mL volumetric flask after cooling, dilute to the mark, and shake well spare.
[0089] 3) Separation and titration
[0090] Separate 5.00ml of the solution into a 300ml Erlenmeyer flask, add 70ml of boiling water, 10mL of buffer solution with pH=10, 1.5g of Neihei T indicator powder, titrate with 0.01mol / L EDTA standard solution to blue as the end point, record EDTA Amount of standard solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com