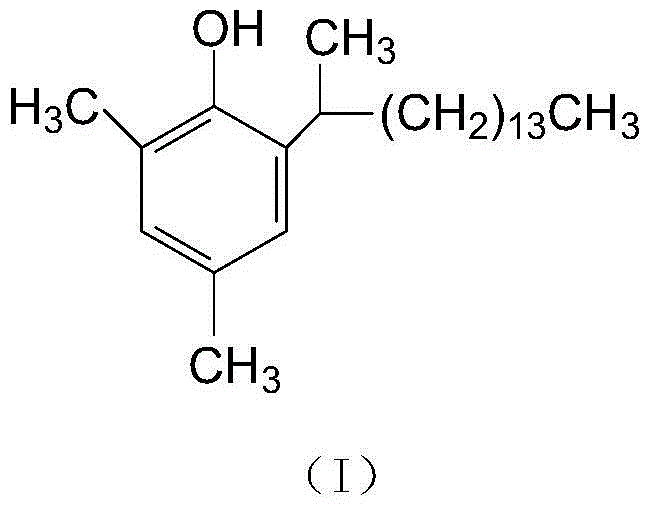
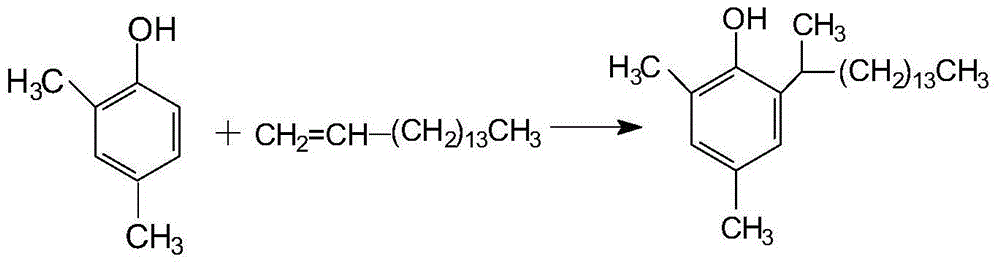
Preparation method of 2, 4-dimethyl-6-(1-methyl-pentadecyl) phenol
A technology of dimethylphenol and pentadecyl, which is used in the field of polymer material additives, can solve the problems of strong corrosion, high reaction temperature, and poor heat resistance, and achieve the effects of less side reactions and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation process of activated carbon-supported sulfuric acid catalyst is as follows: take 50 g of 30-40 mesh fruit shell granular activated carbon, wash it repeatedly with absolute ethanol, and then dry it, add 300 g of concentrated sulfuric acid with a concentration of 98%, stir at room temperature for 1 h, then let stand for 24 h, filter, and Dry and activate at 120°C for 4h, cool down, wash with distilled water until neutral, then dry at 120°C for 2h, the number of acid centers detected is 3.2mmol / g, and set aside.
Embodiment 2
[0022] The preparation process of activated carbon-supported sulfuric acid catalyst is as follows: take 50 g of 30-40 mesh fruit shell granular activated carbon, wash it repeatedly with absolute ethanol, and then dry it, add 200 g of sulfuric acid with a concentration of 80%, stir at room temperature for 1 h, then let it stand for 24 h, filter, and dry it at 120 Dry at 130°C for 2h, then activate at 130°C for 4h, cool, wash with distilled water until neutral, then dry at 120°C for 2h, the number of acid centers is 3.0mmol / g, and set aside.
Embodiment 3
[0024] The preparation process of activated carbon-supported sulfuric acid catalyst is as follows: take 50 g of 30-40 mesh fruit shell granular activated carbon, wash it repeatedly with absolute ethanol, and then dry it, add 260 g of sulfuric acid with a concentration of 90%, stir at room temperature for 1 h, then let it stand for 24 h, filter, and dry at 120 Dry at 140°C for 2h, then activate at 140°C for 4h, cool, wash with distilled water until neutral, then dry at 120°C for 2h, the number of acid centers is 3.6mmol / g, and set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com