Application of compound in tumor resistance
A technology of compounds and uses, applied in the field of medicine, can solve problems such as side effects, and achieve the effect of promoting apoptosis and blocking the cell cycle of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
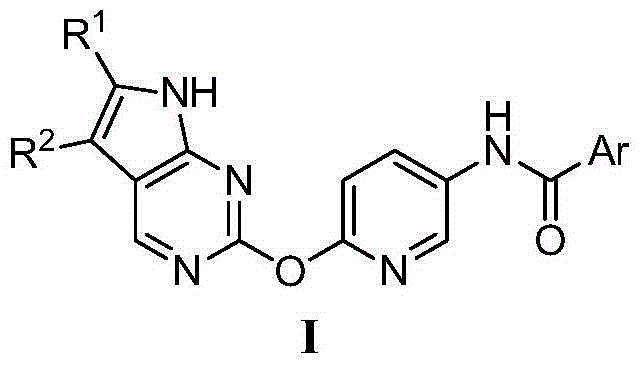
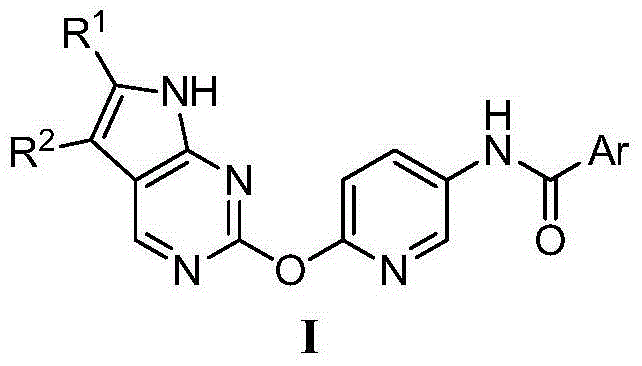
[0033] Preparation of N-(3-(6-(2-7H-pyrrolo[2,3-d]pyrimidinyl)oxy)pyridyl)benzamide (I-1)
[0034] (1) Preparation of 2-((2-5-nitropyridyl)oxy)-7H-pyrrolo[2,3-d]pyrimidine
[0035] Add 15.3g (100mmol) of 2-chloro-7H-pyrrolo[2,3-d]pyrimidine, 15.4g (110mmol) of 2-hydroxy-5-nitropyridine, 65.2g (200mmol) of cesium carbonate into a 500ml three-necked flask ), cuprous iodide 0.2g and anhydrous DMF200ml. The temperature was raised to 75°C and stirred for 3 hours, then cooled to room temperature, the reaction solution was slowly poured into 1000ml of water, and a large amount of gray solid was precipitated. Stand still, filter with suction, wash the filter cake with 50ml of water, and dry under vacuum at 60°C to obtain 22g of 2-((2-5-nitropyridyl)oxy)-7H-pyrrolo[2,3-d]pyrimidine. The rate is 86%. MS [M+H] + 258.1.
[0036] (2) Preparation of 2-((2-5-aminopyridyl)oxy)-7H-pyrrolo[2,3-d]pyrimidine
[0037] Add 2-((2-5-nitropyridyl) oxygen group)-7H-pyrrolo[2,3-d]pyrimidine 22g, 10...
Embodiment 2
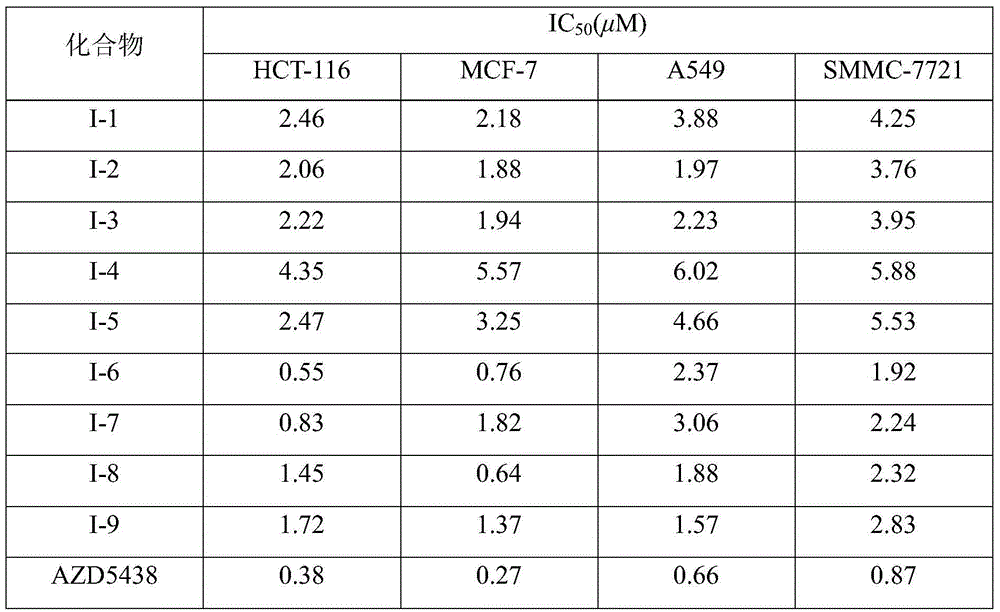
[0043] Test of the inhibitory activity of compound (I-1)~compound (I-9) on CDK2
[0044] The inhibitory activity of the compound of the present invention on CDK2 / cyclin A kinase was tested by fluorescence resonance energy transfer (FRET), and the positive control drug was AZD5438.
[0045] Specific test method: CDK2 / cyclin A is used after diluting to an appropriate concentration with kinase diluent. The kinase reaction mixture contains CDK2 / cyclin A, peptide substrate, HEPES (pH7.5), BRIJ-35, MgCl 2 and EDTA. CDK2phospho-peptide substrate was used as a 100% phosphorylation control, and no ATP was used as a 0% phosphorylation control. After reacting at room temperature for 1 h, moderately diluted Development Reagent A was added to the reaction system. The reaction was continued for 1 h at room temperature, and Stop Reagent was added to terminate the reaction. The excitation wavelength is 400nm, and the fluorescence intensity at the wavelengths of 445nm (coumarin) and 520nm ...
Embodiment 3
[0049] In vitro tumor cell inhibitory activity test of compound (I-1)~compound (I-9)
[0050] Adopt methyl thiazolyl tetrazolium (methyl thiazolyl tetrazolium, MTT) colorimetric method to test the inhibitory activity of the compound of the present invention to tumor cell proliferation in vitro, the selected cell lines are human colon cancer cell HCT-116, human breast cancer cell MCF-7 , human lung cancer cell A549 and human liver cancer cell SMMC-7721, and the positive control drug is AZD5438.
[0051] During the test, take a bottle of cells in the exponential growth phase and in good condition, digest and count them, adjust to the required density and inoculate them in a 96-well cell culture plate. After culturing for 24 hours, add different concentrations of the compound to be tested, and set a positive control drug at the same time. Group and solvent control group, each drug concentration set 3 parallel wells. After 72 hours of drug action on the cells, discard the culture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com