Preparation method for telmisartan
A technology of telmisartan and methyl, which is applied in the field of preparation of telmisartan, can solve the problems of non-combustible vapor pressure and few application reports of ionic liquids, and achieve mild reaction conditions, convenient recovery and reuse, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
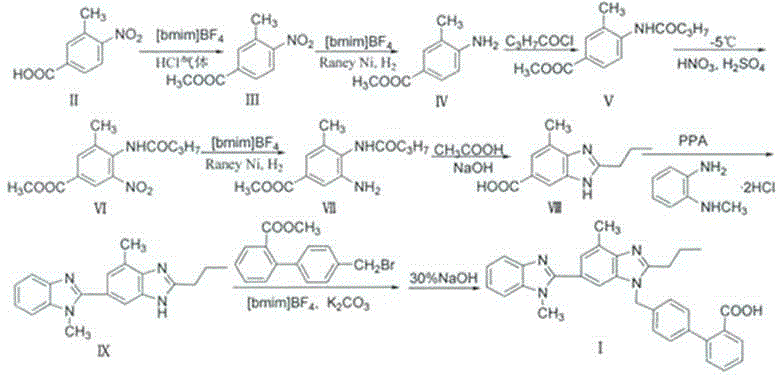
[0036] Synthesis of Telmisartan
[0037]
[0038] (1) Synthesis of 3-methyl-4-nitro-benzoic acid methyl ester Ⅲ
[0039] Compound II (181g, 1.00mol) was added to ionic liquid 1-n-butyl-3-methylimidazolium tetrafluoroborate [bmim]BF 4 (800mL), stir, heat to dissolve, introduce HCl gas (prepared from 120g sodium chloride and 100mL concentrated sulfuric acid), remove the airway after 1h, maintain the reflux state for 6h, cool to room temperature, pour into 2500mL ice water, wash with saturated carbonic acid The sodium solution was adjusted to pH = 8, filtered, washed with water until neutral, and dried to obtain light yellow solid III (180 g, yield of 3-methyl-4-nitro-benzoic acid methyl ester 92.3%). Ionic liquid 1-n-butyl-3-methylimidazolium tetrafluoroborate [bmim]BF 4 Recrystallized, mp 81-83°C.
[0040] (2) Synthesis of 3-methyl-4-amino-benzoic acid methyl ester IV
[0041] Compound III (130g, 0.67mol), ionic liquid 1-n-butyl-3-methylimidazolium tetrafluoroborate [bmi...
Embodiment 2
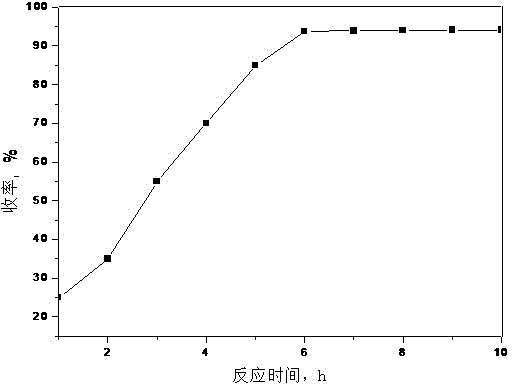
[0056] Effect of Reaction Time on the Yield of Telmisartan
[0057] When telmisartan is synthesized in ionic liquid, other conditions are fixed, only the reaction time is changed, and the influence of different reaction times on the yield of telmisartan is investigated, see figure 1 . Depend on figure 1 It can be seen that when the reaction time is short, the yield is low, and when the reaction time is long, the yield does not increase much, the by-products increase, and the product purity decreases (HPLC mass fraction is only 96.3%). The optimal reaction time should be controlled at about 6 hours, the reaction yield is high, the product purity is high (the HPLC mass fraction of the crude product is 99.1%, the HPLC mass fraction of the refined product is 99.96%), and it is more economical.
Embodiment 3
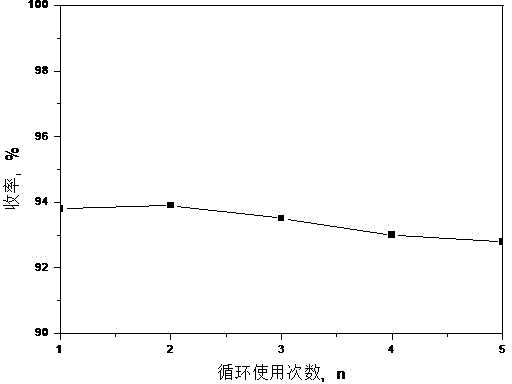
[0059] The Effect of Reuse Times of Ionic Liquids on the Reaction Yield
[0060] Whether the reaction medium used can be recovered and reused is an important content of "green chemistry". This example examines the reusability of ionic liquids for the synthesis of telmisartan. In the ionic liquid used for the synthesis of telmisartan , add compound IX again, add anhydrous potassium carbonate and 4 , -Bromomethylbiphenyl-2-methyl carboxylate, heated to 65°C for reaction, this ionic liquid was reused more than 5 times, the results are shown in figure 2 . Depend on figure 2 It can be seen that after 5 repeated uses of ionic liquids, the yield of the product begins to decrease significantly, which indicates that ionic liquids can be recovered and reused effectively, and the reusable performance is good, which is a kind of recyclable green solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com