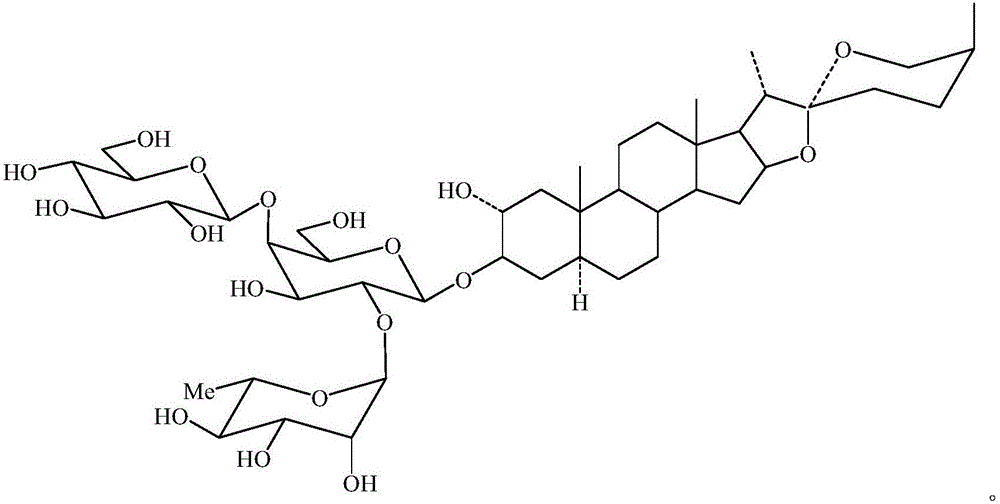
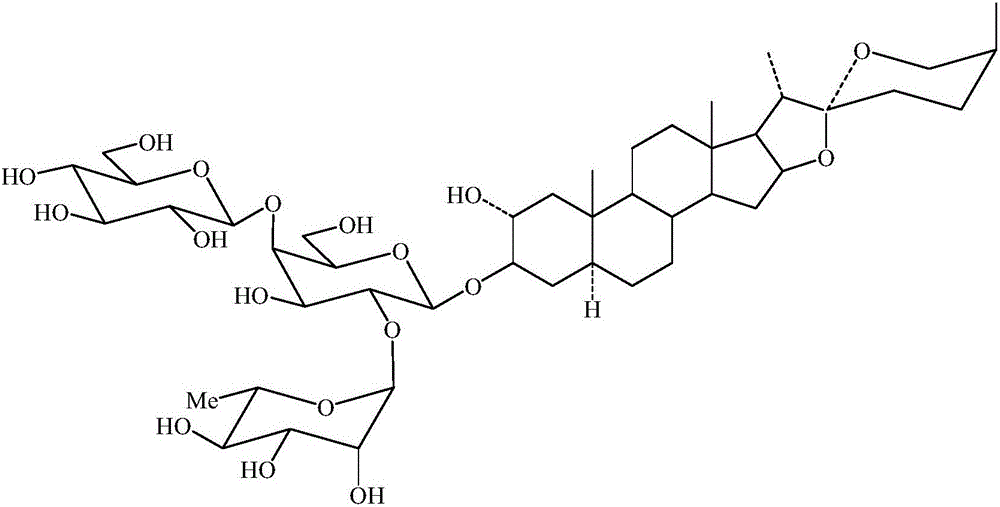
Violaside a and its preparation method
A purple calyx and new glycoside technology, applied in the field of biomedicine, can solve the problems of difficult separation and purification, cumbersome operation, difficult large-scale preparation, etc., and achieve the effect of reducing sample loss, improving separation efficiency and saving separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This embodiment includes the following steps:
[0036] 1) Take 650g of dried purple calyx leaves, pulverize them into coarse powder, and extract with 50% ethanol (v / v) solution (material-to-liquid ratio 1:8) refluxing once for 1 hour each time. The extract is filtered and concentrated to obtain an alcohol dip After 122 g of the alcohol extract was suspended in 1000 mL of water, successively extracted with an equal volume of ethyl acetate, the resulting aqueous layer was extracted with an equal volume of n-butanol, each solvent was extracted once, and finally the n-butanol layer was obtained. The n-butanol is recovered to obtain 27 g of n-butanol extract.
[0037] 2) Take the n-butanol extract obtained in step 1) for chromatography on a silica gel column (loading amount is 1:10), and use a chloroform-methanol solution (100:20~100:40, v / v) gradient elution (diameter The height ratio is 1:6, the flow rate is 3BV / h, 0.2BV is the first batch). The eluent was concentrated under ...
Embodiment 2
[0041] This embodiment includes the following steps:
[0042] 1) Take 652g dried leaves of purple calyx, crush them into coarse powder, and extract twice with 70% ethanol (v / v) solution (material-to-liquid ratio of 1:10) for 2 hours each time. The extract is filtered and concentrated to obtain an alcohol dip Extract 109g of alcohol extract. Suspend the alcohol extract in 1000 mL of water. After successively extracting with equal volume of ethyl acetate, the resulting aqueous layer is extracted with equal volume of n-butanol, each solvent is extracted twice, and finally the n-butanol layer is obtained. The n-butanol is recovered to obtain 25 g of n-butanol extract.
[0043] 2) Take the n-butanol extract obtained in step 1) for chromatography on a silica gel column (loading amount is 1:20), and use a chloroform-methanol solution (100:20~100:40, v / v) gradient elution (diameter The height ratio is 1:8, the flow rate is 6BV / h, 0.2BV is the first fraction), the eluent is concentrated un...
Embodiment 3
[0047] This embodiment includes the following steps:
[0048] 1) Take 650g of dried purple calyx leaves, crush them into coarse powder, reflux and extract 3 times with 90% ethanol (v / v) solution (material-to-liquid ratio is 1:12), each 3h, the extract is filtered and concentrated to obtain alcohol immersion Extract 103 g of the alcohol extract. Suspend the alcohol extract in 1000 mL of water. After successively extracting with an equal volume of ethyl acetate, the resulting aqueous layer is extracted with an equal volume of n-butanol, and each solvent is extracted 3 times. Finally, the n-butanol layer is obtained. The n-butanol is recovered to obtain 28 g of n-butanol extract.
[0049] 2) Take the n-butanol extract obtained in step 1) for chromatography on a silica gel column (loading amount is 1:30), and use a chloroform-methanol solution (100:20~100:40, v / v) gradient elution (diameter The height ratio is 1:10, the flow rate is 9BV / h, 0.2BV is the first fraction), after the eluen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com