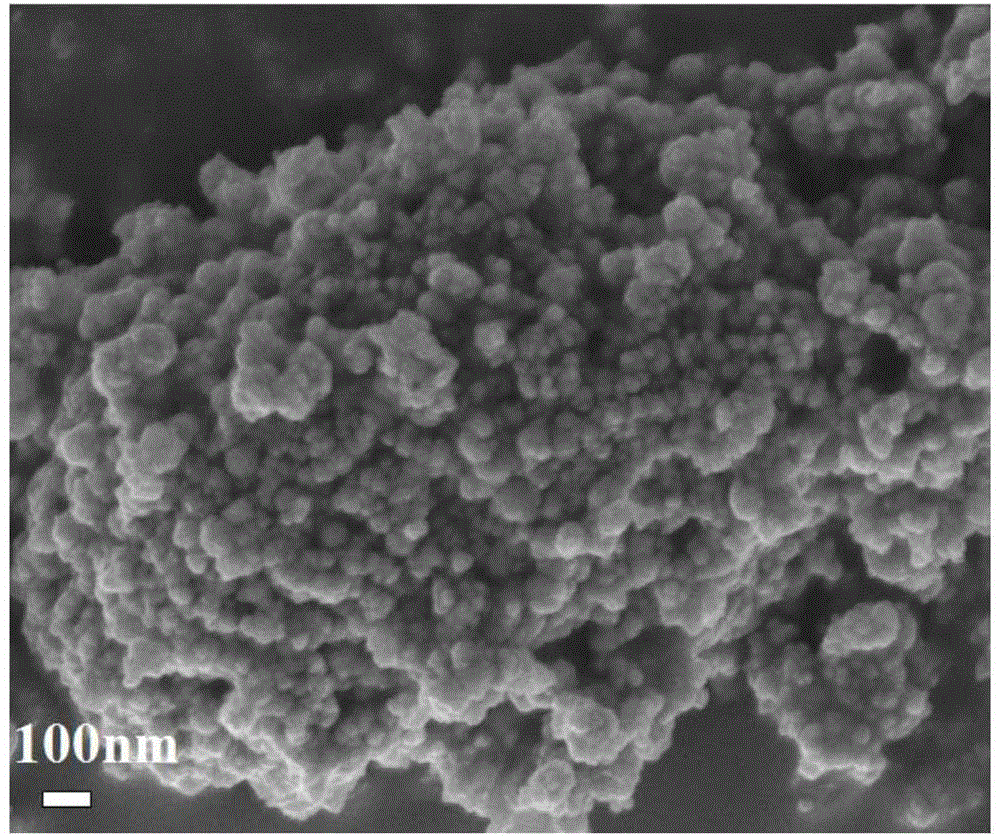
Method for preparing low-carbon olefin catalyst through carbon dioxide hydrogenation
A low-carbon olefin and carbon dioxide technology, which is applied in the production of hydrocarbons from carbon oxides, organic chemistry, and bulk chemical production, etc. Controllable particle size, stable catalyst and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
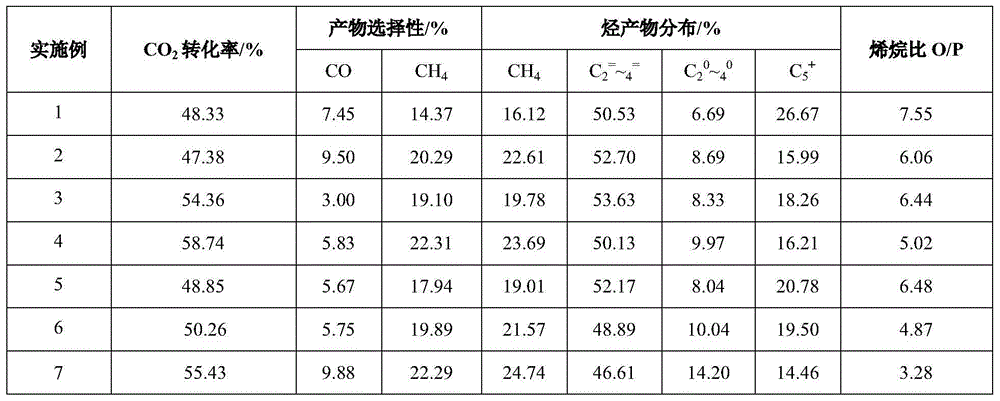
[0026] Weigh 40.4g Fe(NO 3 ) 3 9H 2 O and 54.0g urea are mixed into 200mL homogeneously mixed solution, wherein urea / Fe molar ratio is 9, transfer to sample dissolving cup, in microwave 2450MHz, power 500W, 1.6MPa, heat 2 hours, drop to room temperature, filter, wash and precipitate to Neutral, dry at 120°C for 12 hours, and bake at 400°C for 4 hours. Weigh 0.60g K 2 CO 3 , dissolved in 4mL of deionized water, impregnated into 7g of the above sample, dried at 120°C for 12 hours, and granulated at 20-40 mesh to obtain catalyst sample 1, in which zirconium / iron was 0. At a temperature of 400°C and normal pressure, the space velocity is 1000h -1 30% H 2 The catalyst samples were reduced for 8 hours. Then switch to carbon dioxide hydrogenation feed gas (H 2 / CO 2 =3、Airspeed=1000h -1 ), reacted for 100 hours at a temperature of 320° C. and a pressure of 2 MPa. The results are shown in Table 1.
Embodiment 2
[0028] Weigh 40.4g Fe(NO 3 ) 3 9H 2 O, 3.82g ZrO(NO 3 ) 2 2H 2 O and 54.8g urea are mixed with 200mL homogeneous mixed solution, wherein urea / (Fe+Zr) molar ratio is 8, in microwave 2450MHz, power 500W, 1.6MPa conditions heating 2 hours, down to room temperature, filter, wash and precipitate to medium properties, dry at 120°C for 12 hours, and bake at 500°C for 4 hours. Weigh 0.49g K 2 CO 3 , dissolved in 4mL of deionized water, impregnated into 7g of the above sample, dried at 120°C for 12 hours, and granulated at 20-40 mesh to obtain catalyst sample 2, wherein the zirconium / iron ratio was 1:7. At a temperature of 400°C and normal pressure, the space velocity is 1000h -1 30% H 2 The catalyst samples were reduced for 8 hours. Then switch to carbon dioxide hydrogenation feed gas (H 2 / CO 2 =3、Airspeed=1000h -1 ), reacted for 100 hours at a temperature of 320° C. and a pressure of 2 MPa. The results are shown in Table 1.
Embodiment 3
[0030] Weigh 40.4g Fe(NO 3 ) 3 9H 2 O, 5.34g ZrO(NO 3 ) 2 2H 2 O and 43.7g urea are mixed with 200mL homogeneously mixed solution, wherein urea / (Fe+Zr) mol ratio is 6, transfers to the sample dissolving cup, in microwave 2450MHz, power 500W, 1.6MPa condition heating 2 hours, down to room temperature, Filter and wash the precipitate until neutral, dry at 120°C for 12 hours, and bake at 500°C for 4h. Weigh 0.46g K 2 CO 3 , dissolved in 4mL of deionized water, impregnated into 7g of the above sample, dried at 120°C for 12 hours, and granulated at 20-40 mesh to obtain catalyst sample 3, wherein the zirconium / iron ratio was 1:5. At a temperature of 400°C and normal pressure, the space velocity is 1000h -1 30% H 2 The catalyst samples were reduced for 8 hours. Then switch to carbon dioxide hydrogenation feed gas (H 2 / CO 2 =3、Airspeed=1000h -1 ), reacted for 100 hours at a temperature of 320° C. and a pressure of 2 MPa. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com