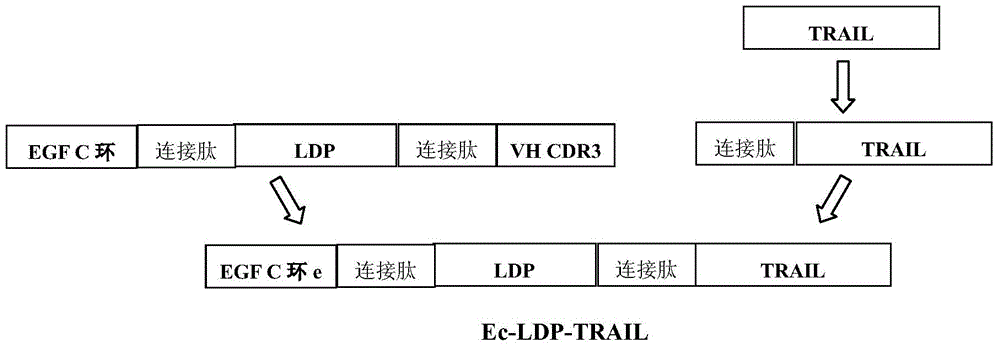
Double-target and double-warhead anti-tumor fusion protein as well as coding gene and application thereof
A fusion protein, coding gene technology, applied in antitumor drugs, peptide/protein components, hybrid peptides, etc., can solve the problems of TRAIL sensitivity difference, TRAIL limitation, etc., and achieve the effect of inhibiting proliferation, inhibiting growth and low drug toxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Construction of fusion protein Ec-LDP-TRAIL recombinant expression plasmid pET30-Ec-LDP-TRAIL
[0139] Recombinant plasmid pET30a(+)-Ec-ldp-Hr (Guo Xiaofang et al., A bispecific enediyne-energized fusion protein containing ligand-based and antibody-based oligopeptides against epidermal growth factor receptor and human epidermal growth factor receptor2shows potent Cactivator Research, 2010, 16.7:2085-2094) and pET30a(+)-TRAIL (Jie Yang, TRAIL combined with LDM and mechanism of action and expression of TRAIL protein. MS thesis. Peking Union Medical College, 2010.) contain Ec-LDP gene and TRAIL functional gene are preserved by our laboratory. Two restriction sites, NdeI and XhoI, were introduced by overlapping PCR technology, and the primers were synthesized by Invitrogen.
[0140] P1 (SEQ ID NO:8): 5'-TATA CATATG AACTGTGTGGTGGGCTATATTGG-3' (the underline is the Nde I restriction site, followed by the Ec-ldp start sequence)
[0141] P2 (SEQ ID NO:9): 5'-CTCAC TGAACC...
Embodiment 2
[0146] fusion protein Ec-LDP-TRAIL in Escherichia coli BL21 (DE3) star TM inducible expression
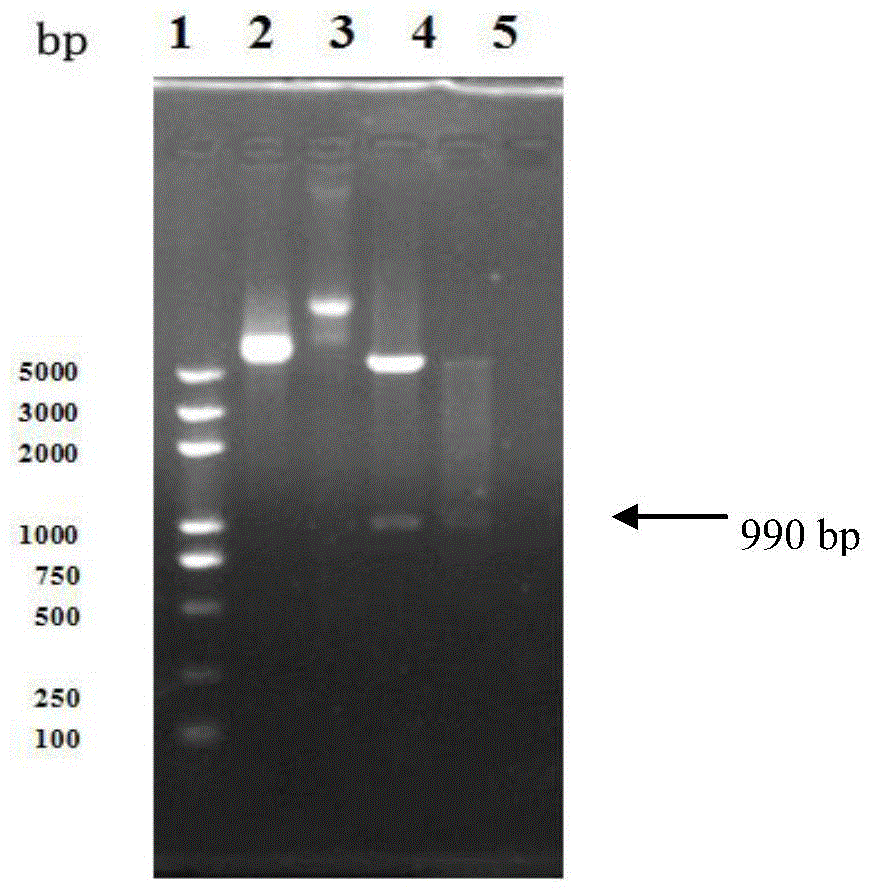
[0147] The identified recombinant plasmid pET30-ec-ldp-trail was transformed into Escherichia coli BL21(DE3)star TM (Invitrogen company product), pick the recombinant transformant (this strain is Escherichia coli), which has been preserved in the General Microorganism Center of China Committee for the Collection of Microorganisms on September 17, 2013 (referred to as CGMCC, address : No. 3, No. 1 Yard, Beichen West Road, Chaoyang District, Beijing, Institute of Microbiology, Chinese Academy of Sciences), strain preservation number CGMCC NO.8200) inoculated into 3ml LB liquid medium containing 50μg / ml kanamycin, 37℃ Incubate overnight with shaking. The next day, they were inoculated at a ratio of 5%, cultured with shaking at 37°C until the OD600 was 0.8, added IPTG to a final concentration of 0.2mM, and induced for 8 hours. An appropriate amount of bacterial liquid was taken, an...
Embodiment 3
[0148] fusion protein Ec-LDP-TRAIL affinity chromatography purification and separation preparation
[0149] 3.1 Extraction of inclusion body protein
[0150]The culture of recombinant bacteria induced by IPTG was centrifuged at 10000 g for 10 min, the supernatant was removed as much as possible, and the bacteria were collected. Resuspend the cells with 20ml of 20mM Tris-HCl per 1L of culture volume. Sonication disrupts cells. Centrifuge at 10,000 g at 4°C for 15 min to collect inclusion bodies and cell fragment proteins, and discard the supernatant. Resuspend the pellet with 40 ml of urea-free 1× binding buffer (20 mM Tris-HCl, 0.5 M NaCl, 5 mM imidazole, pH 8.0). Centrifuge at 10,000 g for 15 min at 4°C, discard the supernatant, and collect the precipitate. Repeat the above steps, resuspend the pellet with 50ml of 1× binding buffer (20mM Tris-HCl, 0.5M NaCl, 20mM imidazole, 8M urea, pH8.0), and ice-bath for 1h to completely dissolve the inclusion body protein. Centrifug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com