Preparation method of antibody-conjugated medicine, antibody-conjugated medicine and applications
A technology of antibody-conjugated drug and monoclonal antibody, which is applied in the preparation of antibody-conjugated drug, antibody-conjugated drug and its application field, and can solve the problem of instability of thiol-maleimide linker and the incompatibility of screening mutation sites , low coupling efficiency and other issues, to achieve the effects of cost savings, good anti-tumor effect, and improved coupling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of anti-CD20 antibody variants.
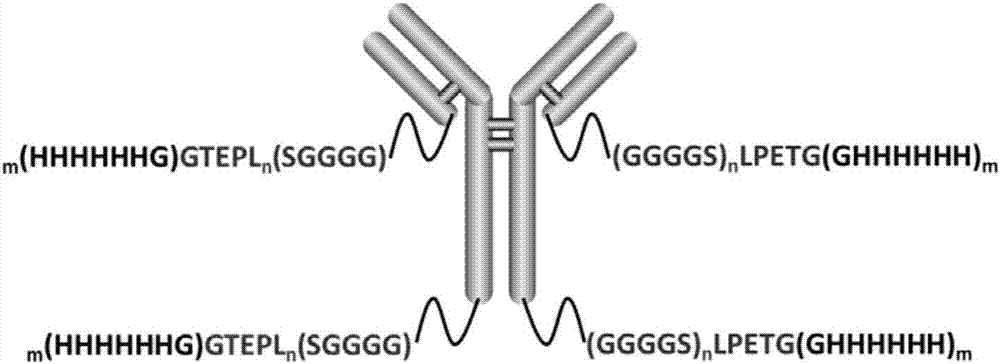
[0044] 1. Construct the expression vector of anti-CD20 antibody with LPETG label for light and heavy chain.
[0045] Ofatumumab (ofatumumab, OFA) is a fully humanized targeting anti-CD20 monoclonal antibody. The gene sequences encoding its heavy chain and light chain are shown in SEQ ID No. 3 and SEQ ID No. 4, respectively.
[0046] The plasmids containing the heavy chain and light chain encoding genes of ofatumumab (heavy chain expression vector H and light chain expression vector L) were obtained by the inventor’s preliminary experiments by PCR technology. For details, please refer to the Chinese application number ZL201310046396.9 Invention patent.) amplified the heavy chain coding gene and light chain coding gene sequence, and then by PCR method to add a signal peptide sequence (nucleotide sequence such as SEQ ID No.5), the C-terminal plus the code (GGGGS) n LPETG(GHHHHHH) m (n = 0, 1, 2; m = 0, 1, a total of 6 combinations) t...
Embodiment 2
[0061] Preparation of anti-CD20 antibody conjugated drugs by enzyme-chemical method.
[0062] 1. Expression and purification of SortaseA enzyme
[0063] For specific steps, refer to Pan, L.Q., et al. (2017) Sortase A-generated highlypotent anti-CD20-MMAE conjugates for efficient elimination of B-lineagelymphomas.Small.13(6).
[0064] 2. Transpeptide reaction catalyzed by SortaseA enzyme
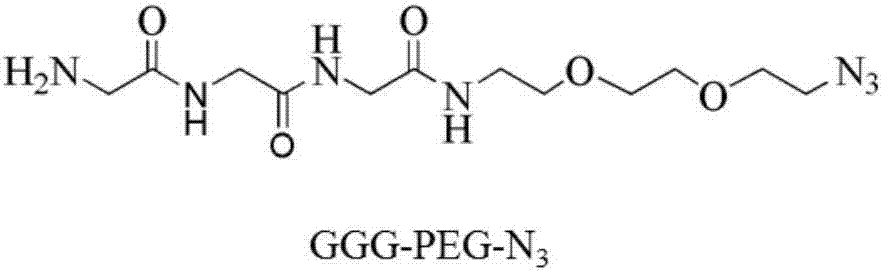
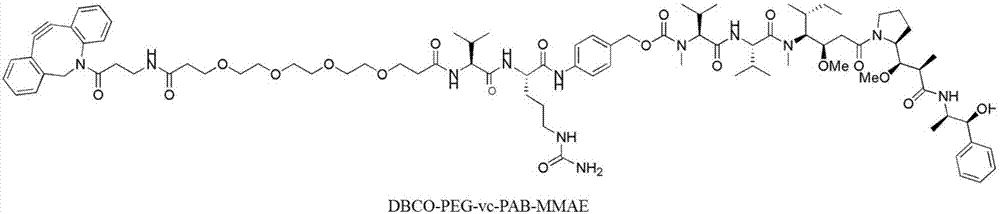
[0065] Bifunctional small molecule GGG-PEG-N 3 (Referred to as GPN) purchased from Nanjing Lianning Biopharmaceutical Co., Ltd., with chemical structure such as figure 2 Shown. In the 50mM Tris, 150mM NaCl (pH7.4) reaction system, respectively add: 2μM recombinant anti-CD20 antibody variant (prepared in Example 1), 50μM Sortase A enzyme, 200μM bifunctional small molecule, 5μM CaCl 2 . Sortase A has a binding site for calcium ions, and the presence of calcium ions will increase the catalytic efficiency of the enzyme. React at 37°C for 12-24 hours. The antibody is catalyzed by SortaseA and the bifu...
Embodiment 3
[0072] The property analysis of anti-CD20 antibody conjugated drugs prepared by enzyme-chemical method.
[0073] 1. RP-HPLC analysis
[0074] Column: Varian PLRP-S column (8μm, 150×25mm); column temperature: 80°C; mobile phase A: 0.1% trifluoroacetic acid aqueous solution; mobile phase B: acetonitrile. Gradient elution conditions: 25% B (3 min); 25-50% B (25 min); 50-95% B (2 min); 95-25% B (1 min); 25% B (2 min). Flow rate: 0.6mL / min; the sample was reduced with DTT 37°C for 30min.
[0075] The result is Image 6 As shown, the anti-CD20 antibody OFA still has an unreduced peak under DTT reduction conditions. The anti-CD20 antibody conjugated drug OFA-GPN-vcMMAE and Shanghai Latoxin have similar light and heavy chain peak times, resulting in a combined peak ( L1+H1), the drug-antibody coupling ratio (DAR) cannot be accurately determined.
[0076] 2. HIC analysis
[0077] The anti-CD20 antibody conjugated drug OFA-GPN-vcMMAE was further analyzed by HIC.
[0078] Chromatographic column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com