Kit for detecting lipoprotein-associated phospholipase A2 and preparation method and application of kit
A phospholipase and lipoprotein technology, applied in the field of biochemical detection, can solve the problems of poor test repeatability, low detection sensitivity, and long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] The following is the preparation method of each component of the kit:
[0091] Preparation 1: Preparation of magnetic sphere suspension of monoclonal antibody or polyclonal antibody coated with Lp-PLA2
[0092] The immunomagnetic spheres used in this preparation step are selected from the nano-magnetic microsphere suspension produced by Merck Company with a concentration of 100 mg / ml and a hydroxyl group of 95 mg KOH / g.
[0093] (1) Preparation of buffer:
[0094] Weigh 2.55g of sodium acetate trihydrate, dissolve it in 4500ml of purified water, add 14ml of acetic acid, and mix well to obtain an acetate buffer solution with a pH of 3.6.
[0095] (2) Magnetic microsphere connection (magnetic microsphere connection CMC method):
[0096] Suspend the magnetic microspheres in the above-mentioned pH3.6 acetate buffer solution 5 times of the coating volume, so that the concentration of the magnetic beads is 20mg / ml; then add 1-cyclohexyl-2-morpholinoethylcarbodiimide to Tos...
Embodiment 1
[0164] Using the first anti-Lp-PLA2 antibody, prepare a suspension of magnetic spheres coated with the first anti-Lp-PLA2 antibody according to Preparation 1 above.
[0165] Using the second anti-Lp-PLA2 antibody, a second anti-Lp-PLA2 antibody solution labeled with ABEI was prepared according to Preparation 4 above.
[0166] Preparation of replacement agent: Measure 1000ml of purified water into a beaker, weigh 15g CHAPS, 0.5g casein, 2g EDTA-2Na, 6g DDT, 1g Tris, 3g MES and 2g NaN 3 Once dissolved in water, after it is completely dissolved, measure and add 150ml of newborn bovine serum, 5ml of acetic acid, 25ml of horse serum, 10ml of glycerol and 25g of BSA, and then filter after fully mixing to obtain the replacement agent solution.
[0167] Preparation of calibrator dilution: Accurately weigh 0.2g KH with an analytical balance 2 PO 4 , 2.9g Na 2 HPO 4 12H 2 O and 8g NaCl, 5g BSA, 2g NaN 3 and 0.125g MgCl 2 , slowly add purified water to adjust the volume to 1000ml,...
Embodiment 2
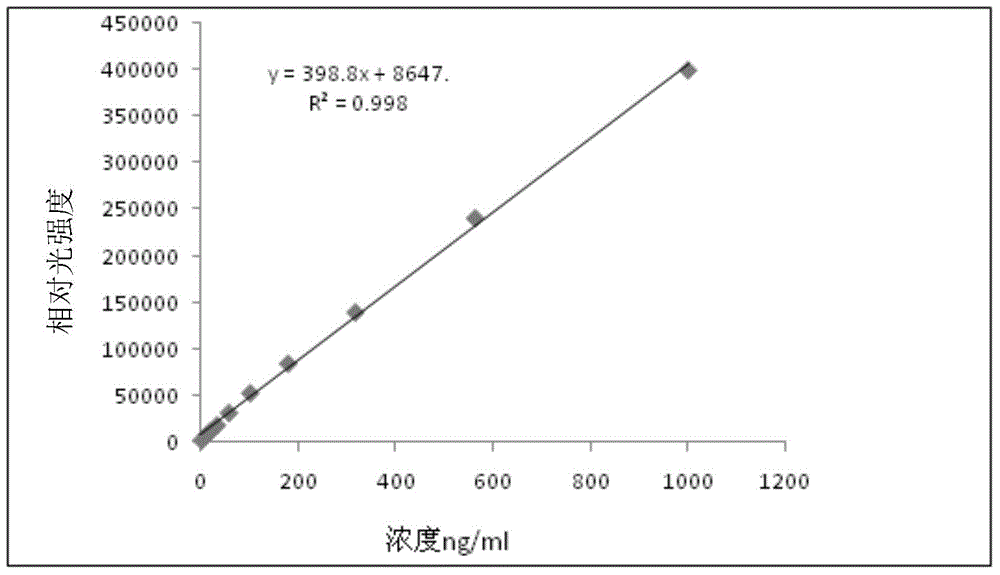
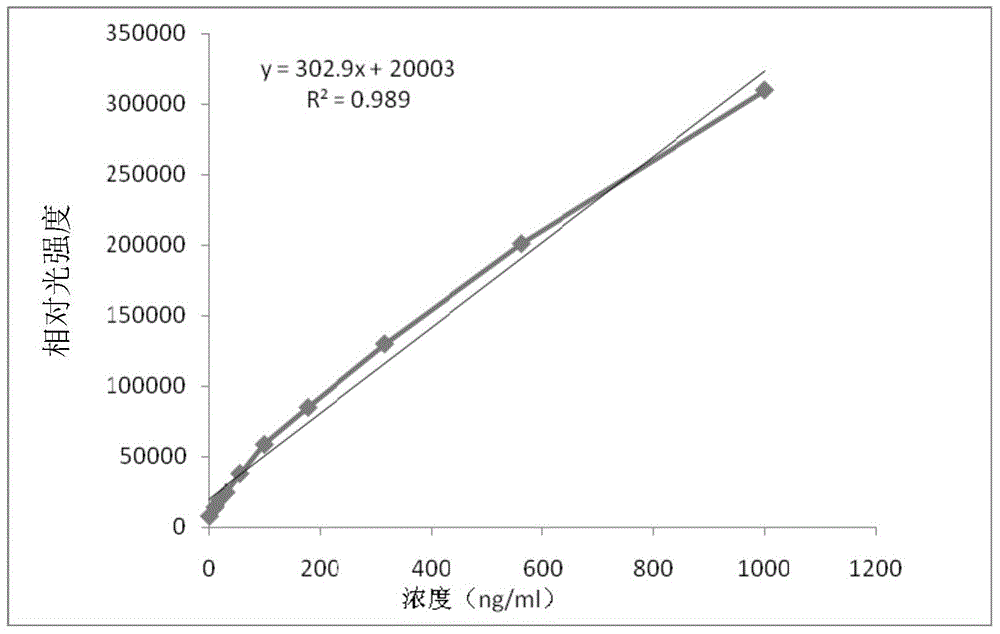
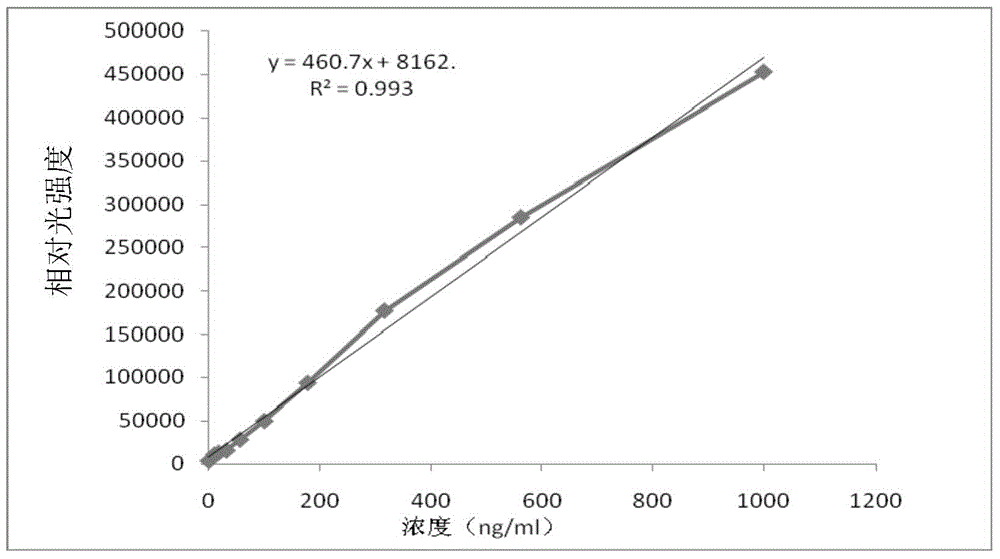
[0181] The composition and detection method of the kit in this implementation are roughly the same as those in Example 1, the only difference being that no displacing agent is used in this example. The results are shown in Table 1-4, figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com