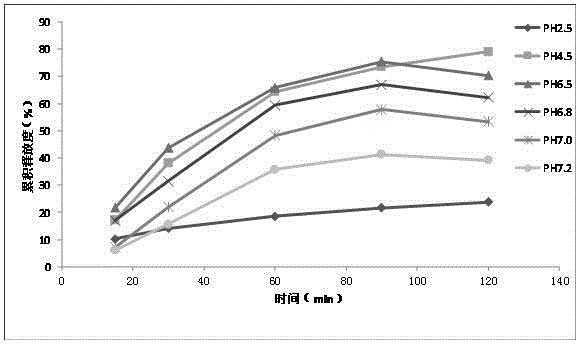
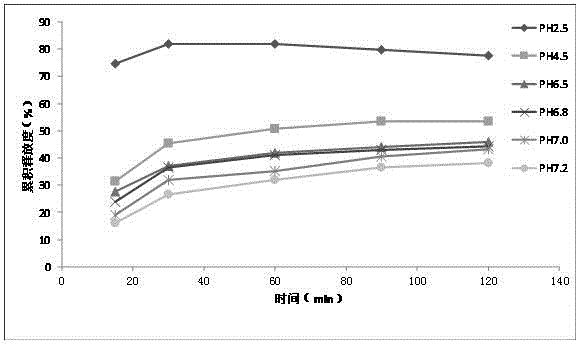
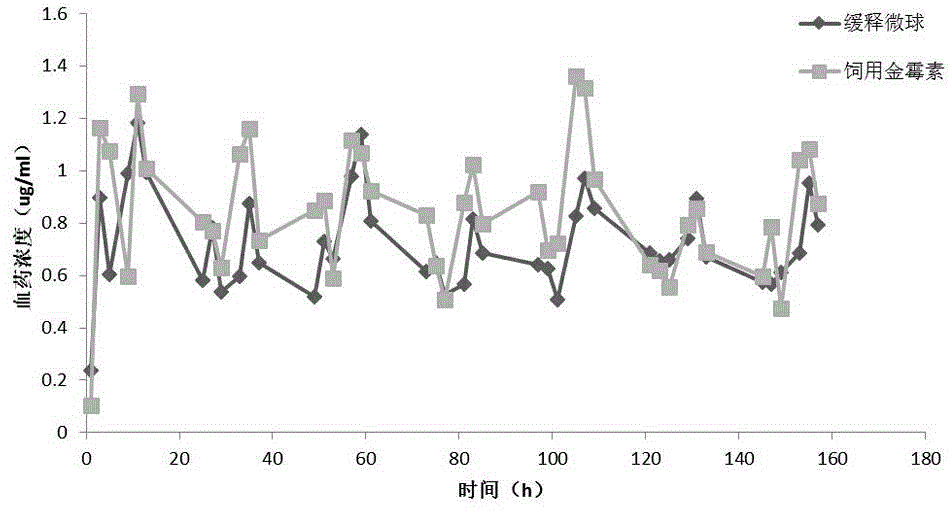
Preparation method of chlortetracycline sustained-release microspheres
A technology for slow-release microspheres and chlortetracycline, which is applied to non-active ingredients in medical preparations, pharmaceutical formulations, active ingredients of tetracycline, etc. The effect of higher viscosity, shorter drying time and higher utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of aureomycin-montmorillonite slow-release microspheres, comprising the steps of:
[0035] S1. Mix 2.0g chlortetracycline and 11.8g montmorillonite evenly, add 2000 parts of water, stir magnetically, and the speed is 200r min -1 , the stirring time is 120min;
[0036] S2. Add 6.0 g of sodium alginate to 4000 parts of water, and heat to prepare a sodium alginate solution at a heating temperature of 60° C.;
[0037] S3. Add the S1 suspension to the S2 sodium alginate solution, stir evenly with a magnetic force, and the speed is 1200r min -1 , the stirring time is 120min;
[0038] S4. Drop the above mixture into 0.2% calcium chloride solution at a rate of 5ml / min for cross-linking for 30min, wash three times with water, soak in absolute ethanol for 90min, filter, collect gel balls, and dry naturally to obtain aureomycin-mongocytidine Destoned slow-release microsphere finished products;
[0039] The molecular weight of the sodium alginate is 12000. ...
Embodiment 2
[0042] A preparation method of aureomycin-montmorillonite slow-release microspheres, comprising the steps of:
[0043] S1. Mix 2.3g chlortetracycline and 11.0g montmorillonite evenly, add 2000 parts of water, stir magnetically, and the speed is 500r min -1 , the stirring time is 90min;
[0044] S2. Add 6.5g of sodium alginate to 4000 parts of water, and heat to prepare a sodium alginate solution at a heating temperature of 90°C;
[0045] S3. Add the S1 suspension to the S2 sodium alginate solution, stir evenly with a magnetic force, and the rotation speed is 1000r min -1 , the stirring time is 120min;
[0046] S4. Drop the above mixture into 3.0% calcium chloride solution at a rate of 8ml / min for crosslinking for 30min, wash three times with water, soak in absolute ethanol for 30min, filter, collect gel balls, and dry naturally to obtain aureomycin-mongocytidine Destoned slow-release microsphere finished products;
[0047] The molecular weight of the sodium alginate is 8000....
Embodiment 3
[0050] A preparation method of aureomycin-montmorillonite slow-release microspheres, comprising the steps of:
[0051] S1. Mix 2.8g chlortetracycline and 10.3g montmorillonite evenly, add 2000 parts of water, stir magnetically, and the speed is 500r min -1 , the stirring time is 90min;
[0052] S2. Add 6.5g of sodium alginate to 4000 parts of water, and heat to prepare a sodium alginate solution at a heating temperature of 70°C;
[0053] S3. Add the S1 suspension to the S2 sodium alginate solution, stir evenly with a magnetic force, and the rotation speed is 1000r min -1 , the stirring time is 120min;
[0054] S4. Drop the above mixture into 2.0% calcium chloride solution at a rate of 10ml / min for crosslinking for 30min, wash three times with water, soak in absolute ethanol for 45min, filter, collect gel balls, and dry naturally to obtain aureomycin-mongocytidine Destoned slow-release microsphere finished product;
[0055] The molecular weight of the sodium alginate is 500...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com