Medicine for treating keratoconus
A keratoconus and pharmacological technology, applied in drug combinations, sensory diseases, pharmaceutical formulations, etc., can solve the problems of patients with great economic and physical and mental impact, cloudy implants, and missed treatment opportunities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Effects of adding different concentrations of 5'aza on antioxidant genes in keratoconus stromal cells
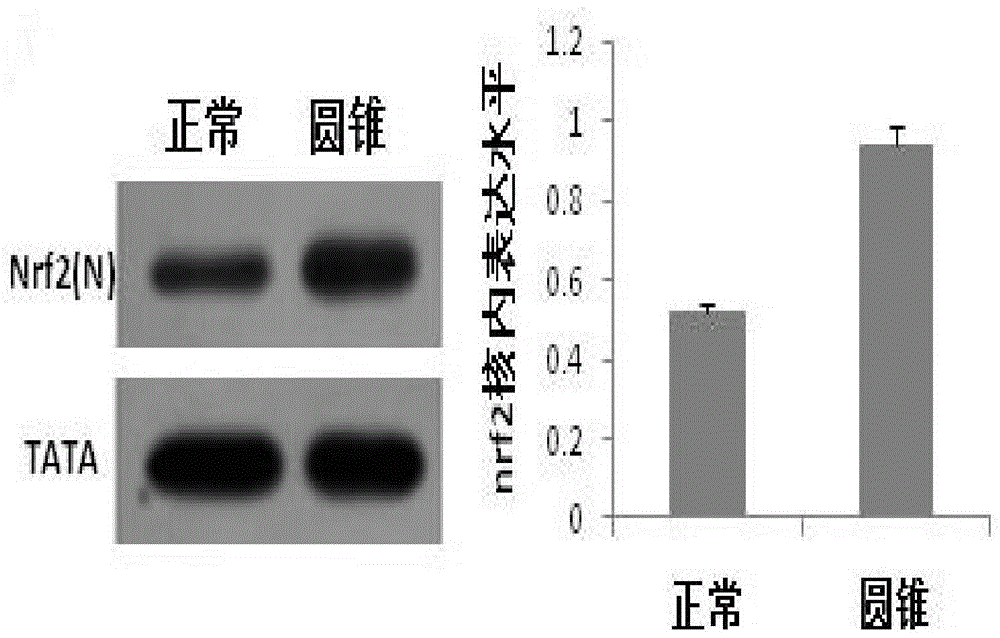
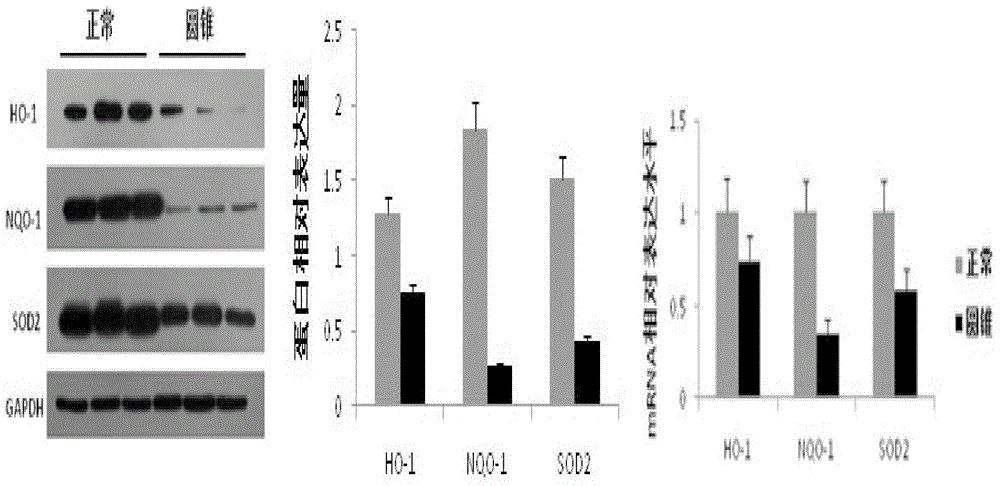
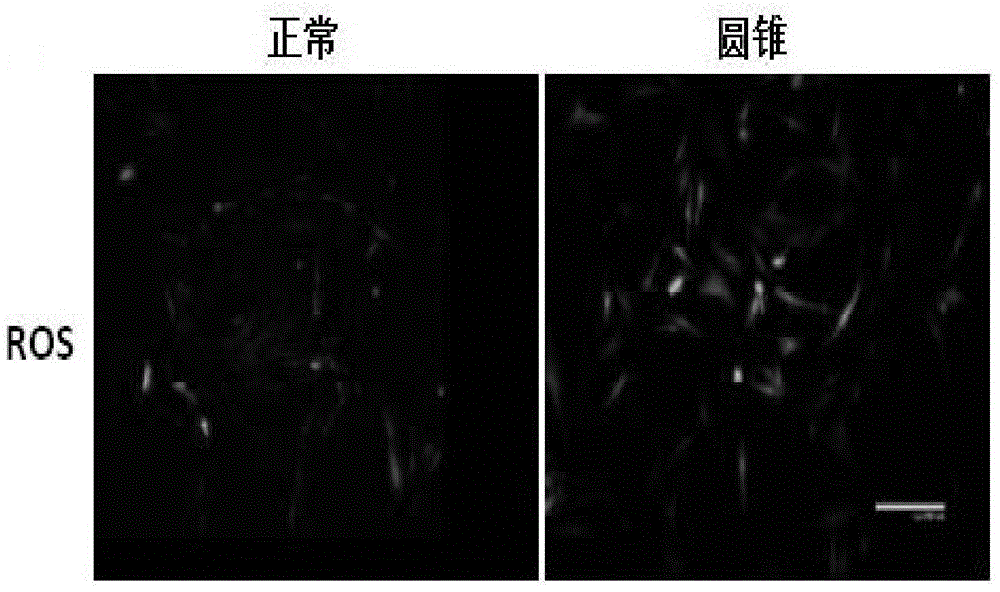
[0019] Corneal tissue was treated overnight at 4°C with Dispase (50mg / ml) to remove the corneal epithelium and endothelium. After the corneal stroma was cut into pieces, it was digested with collagenase (1.25mg / ml) at 37°C for 6-8 hours, separated by pipetting, centrifuged, and the supernatant was discarded. , add DMEM / F-12 medium containing 10% fetal bovine serum at 37°C CO 2 Cultured in an incubator, 0.25% trypsin + 0.02% EDTA for passage. During the isolation and culture of corneal stromal cells, the P3 generation cells were taken 1X10 6 The nuclear protein was extracted according to the steps of the nuclear protein-cytoplasmic protein extraction kit (Thermo, 78833), and the expression of Nrf2 protein in the nucleus of normal and keratoconus stromal cells was detected by wetern blot. The expression level of nrf2 gene in the nucleus of keratoconus sampl...
Embodiment 2
[0022] Example 2: Effect of adding different concentrations of 5'aza on the expression of matrix degrading enzymes in keratoconus stromal cells
[0023] During the isolation and culture of keratoconus stromal cells, different concentrations of methylation inhibitor 5’aza (0, 0.5, 1 μm) were added, and after 48 hours of culture, 5×10 cells were taken. 5 , conventional western blot detection of the protein expression of matrix degrading enzyme UPA in keratoconus stromal cells. After 5'aza treatment, the expression of uPA protein in keratoconus stromal cells was lower than that of the untreated group, such as Figure 5 . In addition, collagen degradation experiments were used to add culture solutions containing plasminogen (100 mg / L) and different concentrations (0, 0.5, 1 μm) of 5'aza to the surface of the three-dimensional collagen gel of keratoconus stroma, cultured for 48 hours, and determined The hydroxyproline content in the solution is used as the collagen degradation ac...
Embodiment 3
[0024] Example 3: The effect of adding different concentrations of SFN on the expression of matrix-degrading enzymes in keratoconus stromal cells
[0025] During the isolation and culture of keratoconus stromal cells, different concentrations of SFN (0, 5, 10 μM) were added, and after 24 hours of culture, 1X10 6 The nuclear protein was extracted according to the procedure of the nuclear protein-cytoplasmic protein extraction kit (Thermo, 78833), and the expression level of Nrf2 protein in the nucleus of keratoconus stromal cells after drug treatment was detected by wetern blot. In addition, take the above cells 5X10 5 , conventional western blot detection of the protein expression of matrix degrading enzyme UPA in keratoconus stromal cells. After SFN treatment, the expression of Nrf2 protein in the nucleus of keratoconus stromal cells increased; and after drug treatment, the expression of UPA protein in keratoconus stromal cells was lower than that in the untreated group. In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com