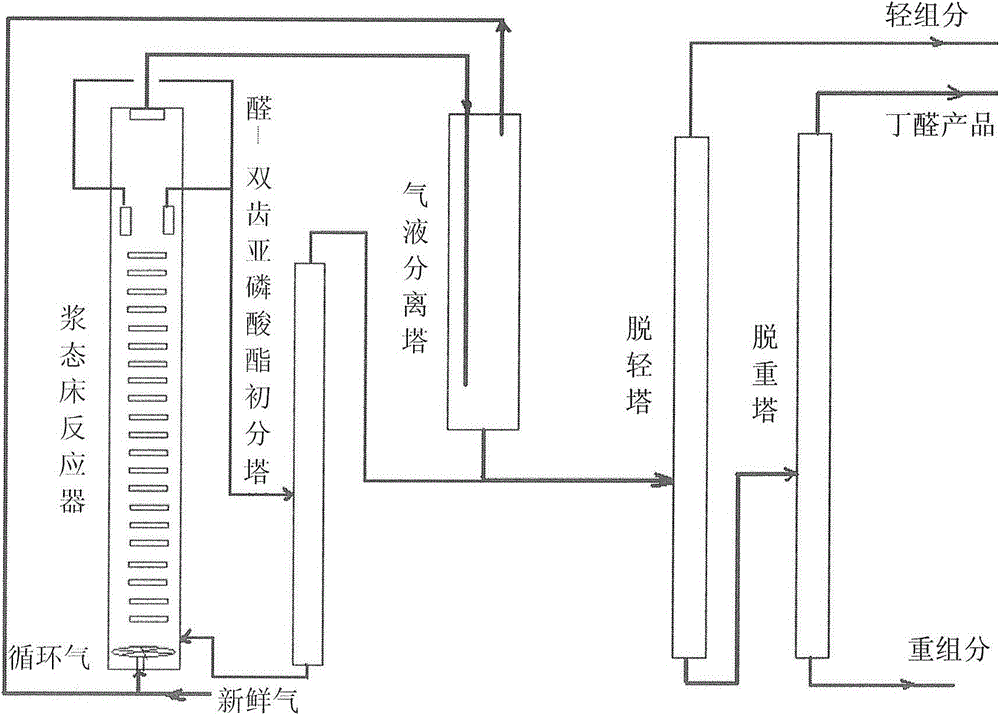
Catalyst system for preparing butyraldehyde by propylene hydroformylation and use method of catalyst system
A technology for producing butyraldehyde and catalyst by hydroformylation of propylene is applied in the directions of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical instrument and method, etc. Problems such as high pressure and temperature, low catalyst activity, etc., achieve the effects of easy recovery, stable performance and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
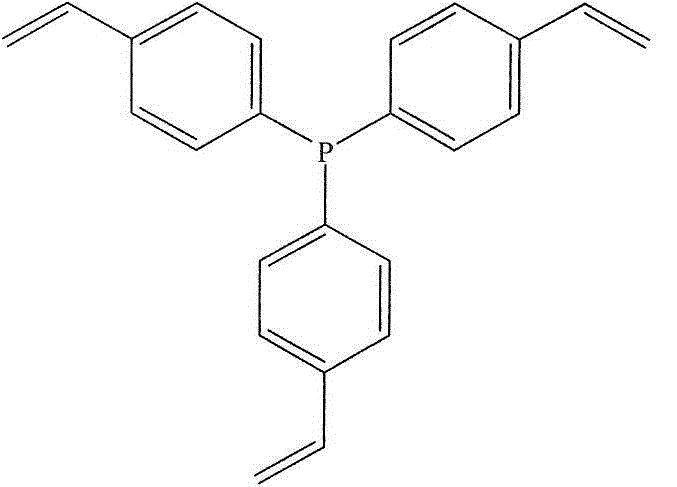
[0051] Under 298K and a nitrogen atmosphere, 100.0 grams of tris(4-vinylphenyl) phosphine ligand monomer was dissolved in 1000.0 ml of tetrahydrofuran solvent, and 10.0 grams of free radical initiator azobisisobutyronitrile was added to the above solution. Stir for 2 hours. The stirred solution was allowed to stand for 24h under 373K and nitrogen atmosphere. The solution after the above-mentioned standing is cooled to room temperature, and the solvent is vacuumed out at room temperature to obtain a P-containing complex with a hierarchical pore structure formed by solvothermal polymerization of tris(4-vinylphenyl) phosphine. Body polymer carrier. The technical route of tris(4-vinylphenyl) phosphine ligand polymer carrier polymerization in this embodiment is as follows:
[0052]
[0053] Here, the degree of polymerization n is in the independent range of 450-550.
[0054] The specific surface area and pore size distribution of the sample were measured on the Quantachrome Instrumen...
Embodiment 2
[0062] Except that the bidentate phosphite cocatalyst L2 was synthesized according to the following steps, the other processes were the same as in Example 1.
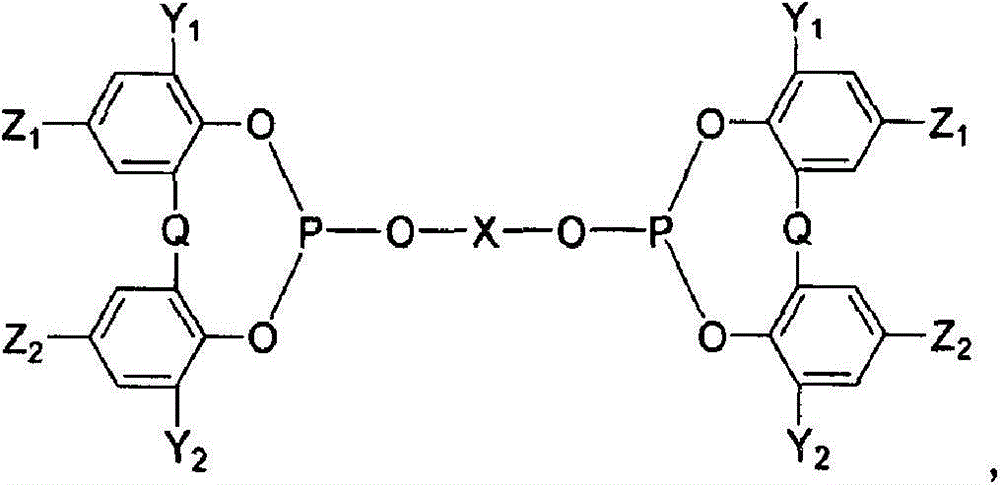
[0063] The synthetic route of L2 bidentate phosphite is as follows:
[0064]
[0065] Add compound B (71.6g, 20mmol) and triethylamine (280ml, 2mol) in tetrahydrofuran solution dropwise to PCl in an ice water bath 3 In the tetrahydrofuran solution (17ml, 200mmol), after the dropwise addition is completed, the temperature is slowly raised to reflux, and after stirring for 2 hours, the solvent is removed under reduced pressure to obtain compound C. Add triethylamine (60ml, 400mmol) in toluene solution (500ml), add dropwise to the toluene solution (1000ml) of compound D (21.6g, 80mmol) and triethylamine (6ml, 40mmol) cooled in an ice-water bath at 0°C , A white precipitate formed. Heated to 80°C and stirred overnight, purified by silica gel column to obtain bidentate phosphite cocatalyst L2. The results of the reaction are sh...
Embodiment 3
[0067] Except that 47.5 grams of iridium acetylacetonate (III) was used in the preparation of the catalyst instead of rhodium acetylacetonate (I), the other processes were the same as in Example 1. The results of the reaction are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com