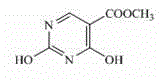
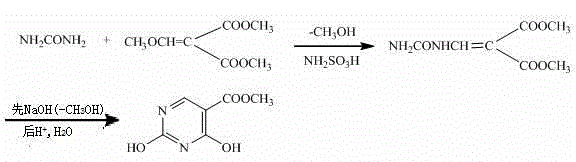
Preparation method of 2,4-dyhydroxyl-5-pyrimidinecarboxylic acid
A technology of pyrimidinecarboxylic acid and dimethyl methoxymethylenemalonate, which is applied in the field of preparation of important organic synthesis intermediate 2,4-dihydroxy-5-pyrimidinecarboxylic acid methyl ester, and can solve problems such as unpublished , to achieve the effect of easy-to-obtain raw materials, simple operation process and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of Dimethyl Methoxymethylene Malonate by Condensation Reaction
[0019] Add 34.8g (0.2mol) of dimethyl methoxymethylenemalonate and 14.4g (0.24mol) of urea into a four-necked flask, add 200mL of ethanol as a solvent, and start stirring to dissolve. Add 3.9g (0.04mol) of sulfamic acid as a catalyst to the four-neck flask, heat and reflux for 5 hours, distill off the solvent, cool, vacuum filter, rinse the filter cake with ice water for several times, at 80°C Dry to constant weight. 25.6 g of white solid ureidomethylene malonate dimethyl was obtained, the yield was 63.37%, m.p.227~230°C.
[0020] Synthesis of methyl 2,4-dihydroxy-5-pyrimidinecarboxylate from ureidomethylenemalonate through ring-closing reaction
[0021] Take 20.2 g (0.1 mol) of ureidomethylene malonate dimethyl ester obtained in the above steps and 160 mL of 10% NaOH solution, heat up to 95 ° C for 2 hours, stop heating, cool to room temperature, and wash with 20% HCl The solution was fir...
Embodiment 2
[0023] Synthesis of Dimethyl Methoxymethylene Malonate by Condensation Reaction
[0024] Add 34.8g (0.2mol) of dimethyl methoxymethylenemalonate and 14.4g (0.24mol) of urea into a four-necked flask, add 200mL of ethanol as a solvent, and start stirring to dissolve. Add 5.8g (0.06mol) of sulfamic acid as a catalyst to a four-neck flask, heat and reflux for 5 hours, distill off the solvent, cool, vacuum filter, rinse the filter cake with ice water for several times, at 80°C Dry to constant weight. 26.5 g of white solid ureidomethylene malonate dimethyl was obtained, the yield was 65.59%, m.p.227~230°C.
[0025] Synthesis of methyl 2,4-dihydroxy-5-pyrimidinecarboxylate from ureidomethylenemalonate through ring-closing reaction
[0026] Take 20.2 g (0.1 mol) of ureidomethylene malonate dimethyl ester obtained in the above steps and 160 mL of 10% NaOH solution, heat up to 95 ° C for 2 hours, stop heating, cool to room temperature, and wash with 20% HCl The solution was first...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com