Recombinant archaerhodopsin 4 protein as well as preparation method and application thereof
A violin and protein technology, applied in the field of genetic engineering, can solve the problems of prolonged attenuation, short light cycle, difficult to realize optical information storage and processing, etc., and achieve the effect of good spatial folding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Using the wild-type AR4 gene as a template, replace the 1-27 bases at the 5' end of the AR4 gene. (SEQ ID NO: 4) and 5'-GCCAAGCTTCTAGATCAGTCGCTG-3' (SEQ ID NO: 5) as primers, the DNA fragment 1 was obtained by PCR amplification with Pfu DNA polymerase, and then the DNA fragment 1 was used as a template, and the 5'- ATGTTGGAGTTATTGCCAACAGCAGTG-3' (SEQ ID NO: 6) and 5'-GCCAAGCTTCTAGATCAGTCGCTG-3' (SEQ ID NO: 5) were used as primers, and the recombinant ancient rhodopsin 4 nucleotide sequence was obtained by PCR amplification with Pfu DNA polymerase, such as SEQ ID NO.1 is shown.
[0048]Subsequently, the recombinant ancient rhodopsin 4 gene was spliced with the promoter sequence that regulates its expression. The steps are as follows: use the pUC19-bop plasmid containing the target promoter sequence as a template, and use 5'-CGGGATCCGACGTGAAGATGGGG-3' (SEQ ID NO: 7) and 5'-ACTCCAACATGCAACAGTACCTAAC-3' (SEQ ID NO: 8) were used as primers, and the bop promoter fragment w...
Embodiment 2
[0050] The gene mutation described in the present invention is realized by the method provided by the KOD-Plus-Mutagenesis Kit (TOYOBO company), and the specific steps are as follows: using the pUC19-bAR plasmid as a template, two reverse PCR primers are designed. The primers can linearize the template plasmid after PCR amplification, and one of the primers has a codon for the residue to be mutated at the 5' end. Using these two primers, use KOD DNA polymerase to perform PCR amplification on the template plasmid to obtain a 3800bp fragment, which already has a mutation site. The PCR conditions were 94°C, 2min, 98°C, 10sec, 68°C, 4min, 15 cycles. Add DpnI restriction endonuclease to the PCR product, treat at 37°C for 1 hour, and digest the plasmid template. The digested PCR product is phosphorylated by phosphokinase and then self-circularized by ligase. The circularized product was transformed into Escherichia coli Top10 competent cells, several clones were randomly selected ...
Embodiment 3
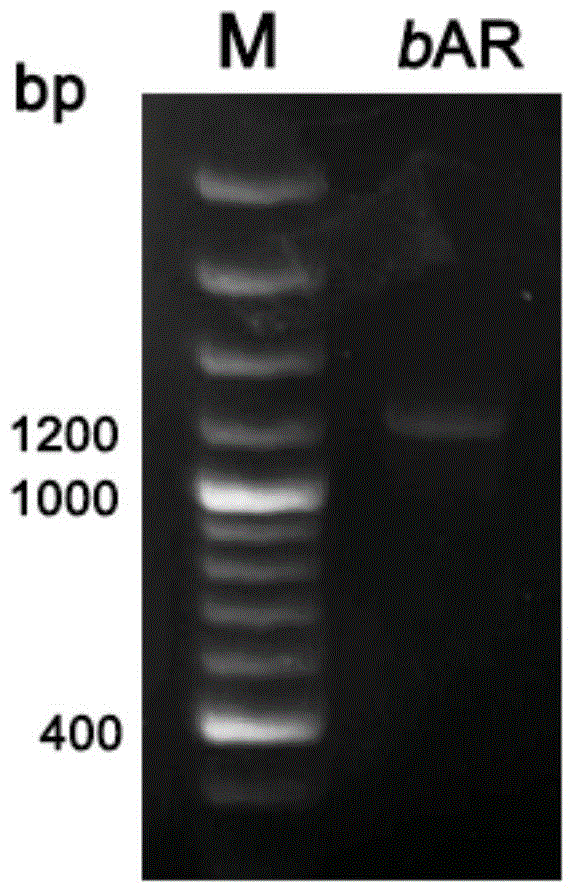
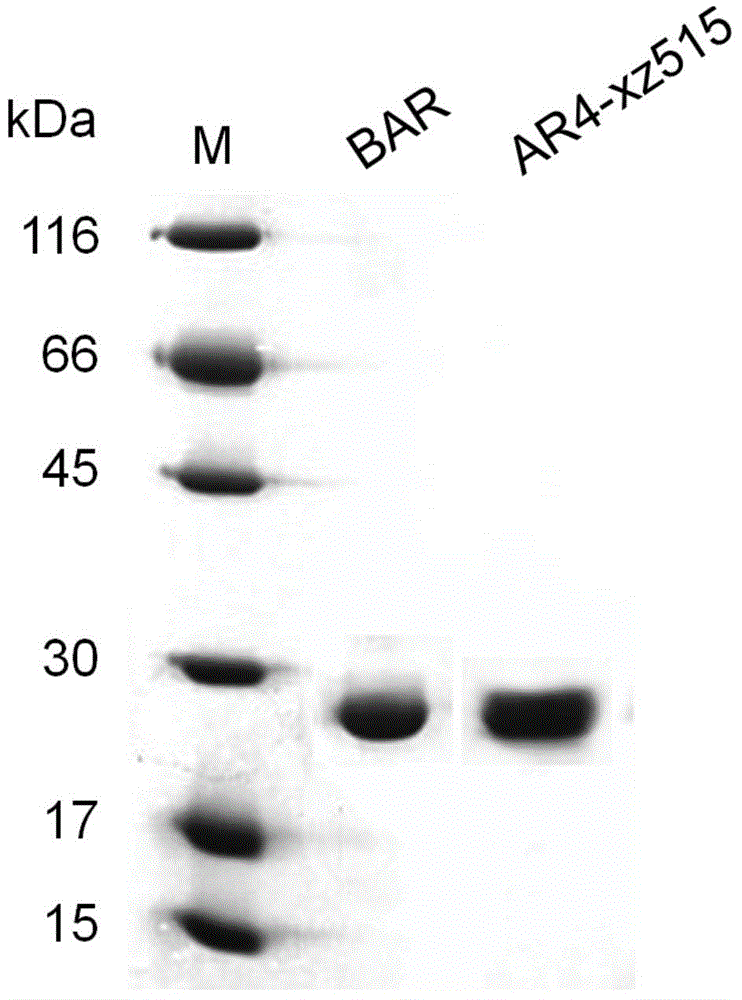
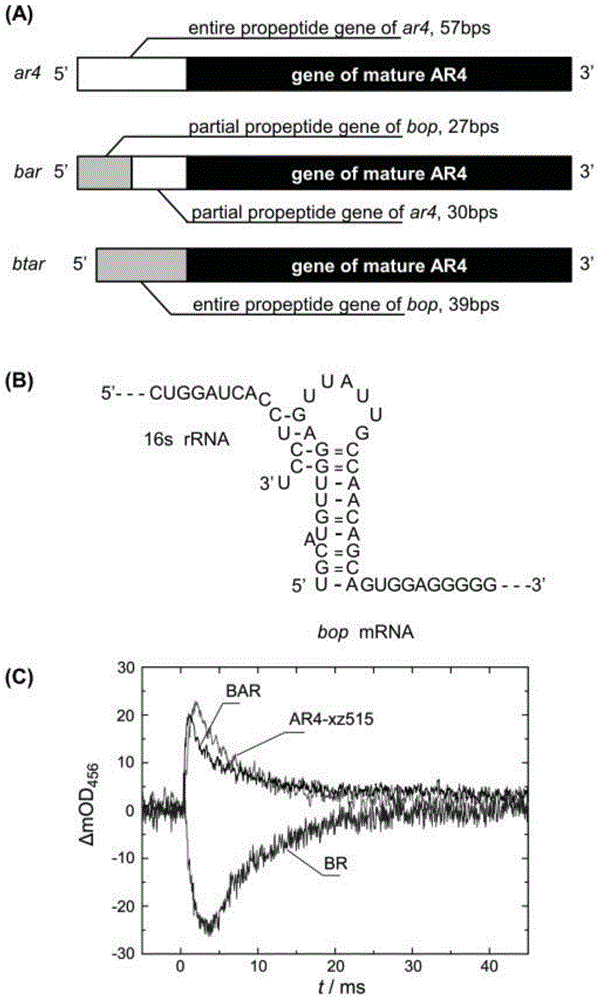
[0052] Using the method described in Example 1, the 5' end of the wild AR4 gene was modified so that the modified gene could express the recombinant rhodopsin 4 protein in a strain that does not produce retinal protein. The total gene length of wild-type AR4 is 789bp, and the first 57 bases encode the leader peptide of AR4 protein. The gene encoding the nine peptides at the N-terminal is replaced with the corresponding part of the bop gene, and the replaced gene is transcribed to obtain mRNA, and its 5' end can form a stem-loop structure to promote translation efficiency ( image 3 B). The modified AR4 gene was spliced with the bop gene promoter, named bAR gene ( image 3 A), and connect the pUC19 plasmid through the BamHI at the 5' end and the HindIII restriction site at the 3' end to form a recombinant plasmid pUC19-bAR, and perform DNA sequencing. Sequencing results showed that its sequence completely matched the sequence of the target gene. The bAR gene cloned on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com