Recombinant avian influenza subunit vaccine and preparation method thereof
A technology of subunits and recombinant antigens, applied in the fields of botanical equipment and methods, biochemical equipment and methods, recombinant DNA technology, etc., can solve the problems of long production cycle and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 1. Plasmid construction of H5 subtype HA gene and NA gene:
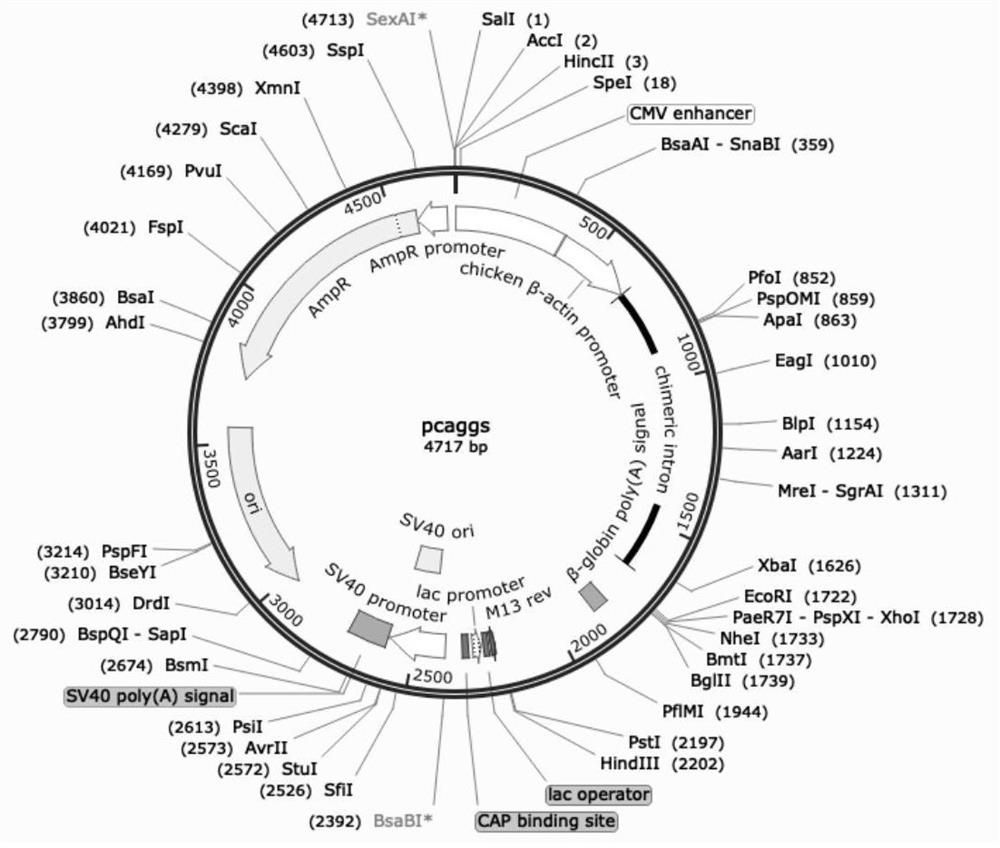
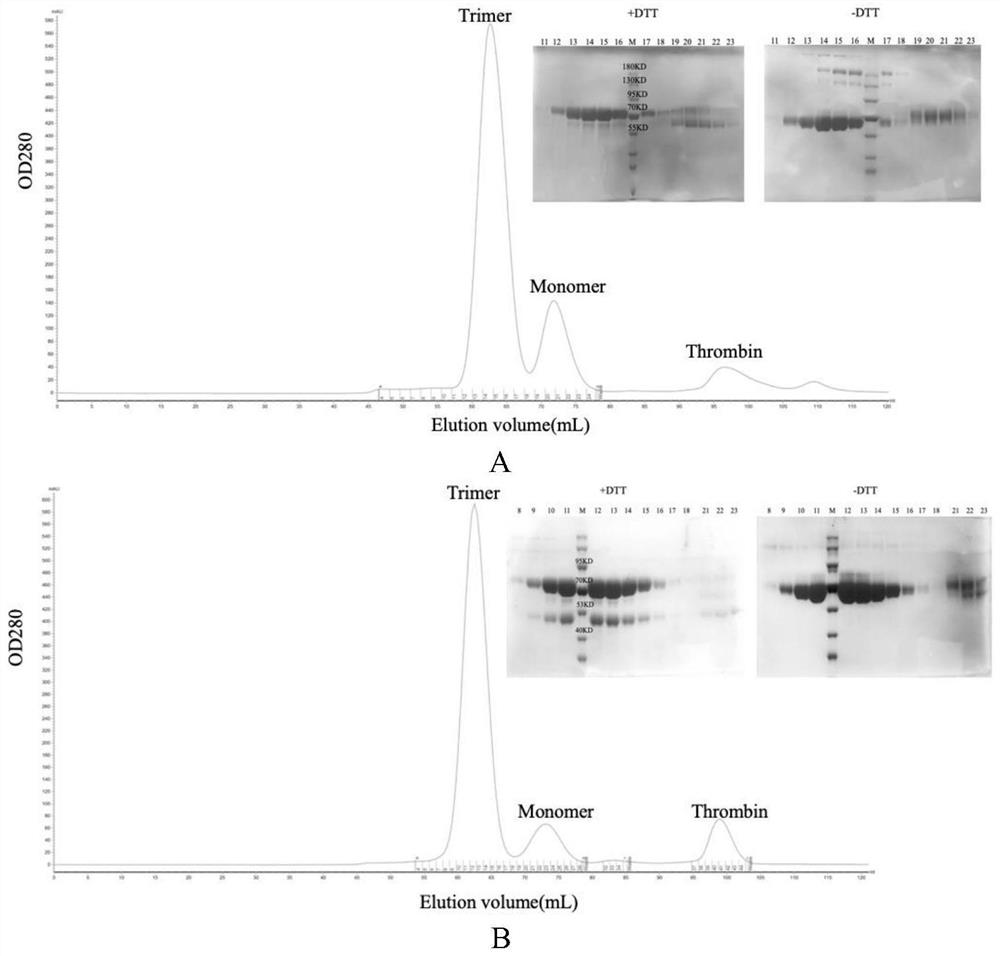
[0085] We double digested the DNA fragment encoding HA or NA (EcoRI and XhoI restriction sites) into the PCAGGS vector (map as figure 1 ), and adding a signal peptide sequence before the coding fragment of the protein is helpful for protein secretion and expression, inserting the coding sequence of 6 histidine tags (hexa-His-tag) and the translation stop codon after the coding fragment of the protein, In this way, a recombinant protein with a histidine tag at the C-terminal will be obtained, which is convenient for subsequent protein purification. The recombinant plasmids were confirmed by PCR, enzyme digestion and sequencing to confirm that the inserted foreign fragments were completely correct. When HA is expressed in mammalian cells, sialic acid will be attached to it, resulting in no ability to bind to receptors. Therefore, NA is also expressed, and the gene of 09NA is selected, and FLAG tag is added for ...
Embodiment 2
[0098] Embodiment 2 H5 / H1-LSQ vaccine preparation
[0099] The protein concentration of H5 / H1-LSQ is 10 mg / ml, dilute the protein with PBS, mix the protein with an equal volume of Freund's complete adjuvant or Freund's incomplete adjuvant, and emulsify completely until the bath water does not dissolve for animal inoculation.
Embodiment 3
[0103] 1. Experimental animals: BALB / c female mice aged 4-6 weeks, 6 in each group, and the way of immunization is intramuscular injection.
[0104] 2. Group settings: ① Negative control (adjuvant MF59); ② Positive control (Influenza virus split vaccine TIV); ③ Experimental group H5 / H1-LSQ (vaccine prepared in Example 2); ④ Experimental group H5 / H1 (the vaccine prepared in Comparative Example 1).
[0105] 3. Experimental steps:
[0106] 3.1 Animal immunity:
[0107] First immunization: the immunization dose of the negative group and the experimental group is 100 μL for each mouse; the immunization dose of the positive group is 100 μL for each mouse.
[0108] The immunization dose of each animal in the experimental group is 20μg / 100μL / animal, dilute the protein with PBS, 6 animals in each group, mix different experimental group proteins with an equal volume of MF59 adjuvant, shake until completely emulsified until the bath water does not melt before use immune to animals.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com